Ideal and Nonideal Solutions
A homogeneous mixture composed of two or more components is called a Solution.
- The component which has thehighest quantity in a solution is the solvent.
- The solvent helps determinethe physical state of the solution.
- A solute is a substance, in a solution, found in a smaller amount.
- The solution that has two components is called aBinary Solution.
- The properties of a solution are further defined on basis of theVapour Pressure, Boiling Point, Freezing Point and other Colligative Properties.
The binary liquid in liquid solutions can be further divided into two terms:
- Ideal Solution
- Non-ideal Solution
Ideal solution
The solutions which follow Raoult's law at all temperatures and concentrations are calledIdeal Solution. During its formation no change in enthalpy or volume takes place. In ideal solutions, the interaction between solute-solute and solvent-solvent molecules same as the interaction between solute and solvent molecules.
An ideal solution should possess the following characteristics:
- It should follow Raoult's law
- No heat or energy released during the formation of the solution means the enthalpy of mixing should be zero. e, ΔH(mix)= 0
- The volume of the solution should be constant means the volume of the solution will be equal to the volume of the components. ΔV(mix)= 0
Practically, no solution behaves as an ideal solution. The substances of similar structure andpolaritymix together to form an ideal solution.
Examples of Ideal Solution
The following examples of nearly ideal solutions:
- Benzene and Toluene
- n-hexane andn-heptane
- Chlorobenzene and Bromobenzene
- Ethyl bromide and ethyl iodide
Nonideal solution
The solutions which do not follow Raoult's law at all concentrations andtemperaturesare termed as non-ideal solutions. It is also termedreal solutions.
The enthalpy of a non-ideal solution does not equal zero and it also changesvolume. For example, whensulphuric acidis mixed in water then heat is released and a volume change is also observed. Thus, it is a non-ideal solution.
Non-Ideal solutions possess the following characteristics:
- It does not follow Raoult's law
- The enthalpy during the formation of the solution is not zero. ΔH(mix),not 0
- The volume after mixing may not be constant. ΔV(mix)not 0.
The non-ideal solutions show deviation from Raoult's law.Based on deviation the non-ideal solutions are of the following two types:
- Positive Deviation
- Negative Deviation
Positive and Negative Deviation from Raoult’s Law
Depending on the deviation of Non-ideal solutions,it can be further classified as:
- Positive Deviation
- Negative Deviation
Positive Deviation from Raoult’s Law
Most of the liquid mixtures form a non-ideal solution.
- In this solution, the interaction between solute and solvent is weaker than the interaction between solute-solute and solvent-solvent. Thus,A – B < A – A or B – B.
- PA> PA0xAand PB> P0BxB(as total vapour pressure, i.e.PA0xA+ P0BxB) is greater than predicted with respect to Raoult’s Law
- Sometimes the partial vapour pressure of a miscible liquid is greater than the expected vapor pressure as per Raoult's law.
- The total vapour pressure will be greater than the corresponding vapor pressure in the case of an ideal solution of the same composition.
- Theboiling pointof such solutions is lowered. This behaviour of a non-ideal solution shows a positive deviation from Raoult's law.
Graphically, it can be represented as:
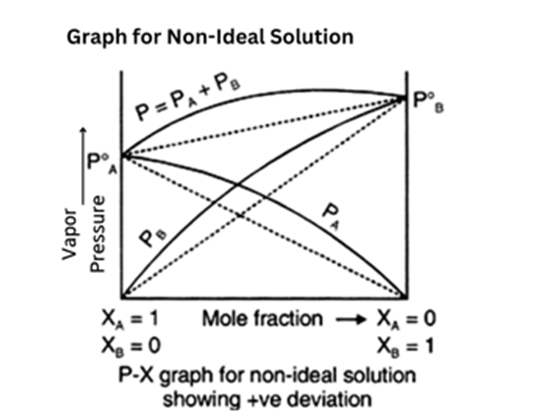
Positive Deviation from Raoult’s Law
The solutions showing positive deviations have an intermediate composition at which the total vapor pressure is maximum, which is greater than the vapor pressure of the pure liquid. The boiling point of such solutions is minimum. This type of solution boils at a constant temperature and gets distilled without changing its composition. Such a mixture is called anAzeotropic mixture.
The non-ideal solutions showing positive deviation are as follows:
- Acetone+ Carbon disulphide
- Acetone + Ethyl Alcohol
- Acetone + Benzene
- Carbon tetrachloride + Chloroform
Negative Deviation from Raoult’s Law
In case of Negative deviation,
- In this solution, the total vapour pressurePA< PA0xAand PB< P0BxB, of the solution, becomes lower than the expected vapour pressure as in Raoult's law.
- Here, the interaction between the solute-solvent molecules is stronger than the solute-solute and solvent-solvent molecules, i.e. A – B > A – A or B – B. This solution is said to exhibit anegative deviationfrom Raoult's law.
- The boiling point of these solutions can be increased with the addition of solute.
Graphically, it can be represented as:
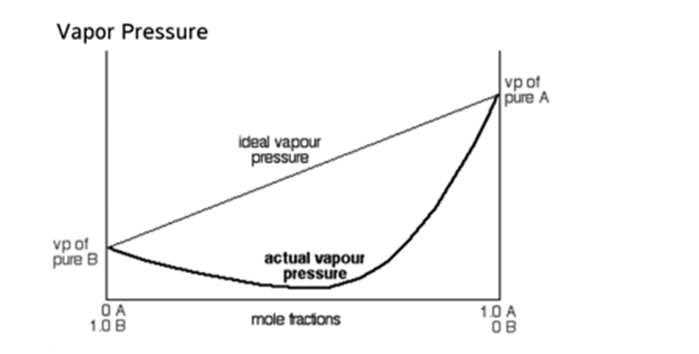
Negative Deviation from Raoult’s Law
Azeotropes
· Some liquids on mixing, form azeotropes which are binary mixtures having the same composition in liquid and vapour phase and boil at a constant temperature.
· An azeotropic mixture cannot be separated into its constituents by distillation (or even by fractional distillation).
Types of azeotropes-
Minimum Boiling Azeotropes
· The solutions which show a large positive deviation from Raoult’s law form minimum boiling azeotrope at a specific composition.
· Example -water and benzene, chloroform and methanol, ethanol – water mixture.
Maximum Boiling Azeotropes
· The solutions that show large negative deviation from Raoult’s law form maximum boiling azeotrope at a specific composition.
· Nitric acid and water is an example of this class of azeotrope.
· This azeotrope has the approximate composition, 68% nitric acid and 32% water by mass, with a boiling point of 393.5 K.