REDOX REACTIONS IN TERMS OF ELECTRON TRANSFER REACTIONS
A few examples of redox reaction on the basis of electronic concept are given below:
According to electronic concept every redox reaction consists of two steps known ashalf reactions.
1. Oxidation reaction:Half reactions that involveloss of electronsare called oxidation reactions.
2. Reduction reactions:Half reactions that involvegain of electrons are called reduction reactions.
Reducing agent
Reducing agents are those compounds which can reduce other and oxidize itself during the chemical reaction. Those reagents in which for an element, oxidation number increases or which undergoes loss of electrons in a redox reaction are termed as reductants.
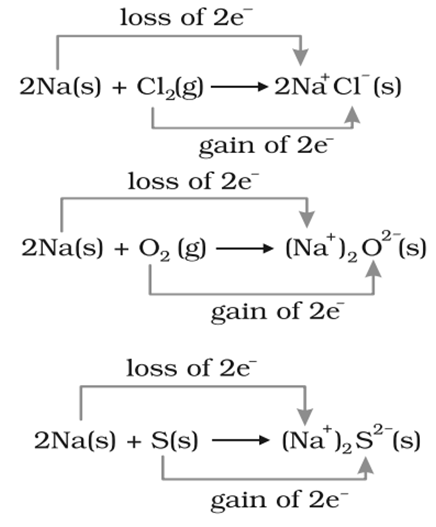
oxidizing agent
Oxidising agents are those compounds which can oxidize others and reduce itself during the chemical reaction. Those reagents in which for an element, oxidation number decreases or which undergoes gain of electrons in a redox reaction are termed as oxidants.
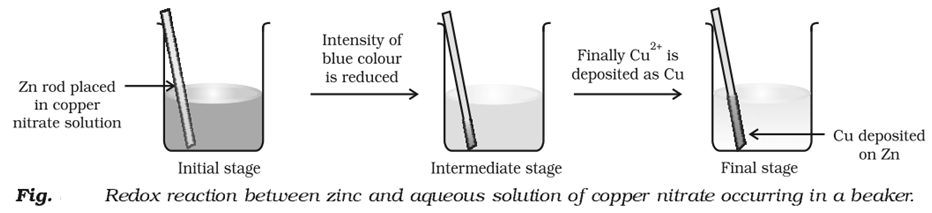
Competitive Electron Transfer Reaction
To understand this concept let us do an experiment.
Place a strip of metallic zinc in an aqueous solution of copper nitrate as shown in Fig. After one hour following changes will be notices.
1. Strips becomes coated with reddish metallic copper.
2. Blue colour of the solution disappears.
3. If hydrogen sulphide gas is passed through the solution appearance of white ZnS can be – seen on making the solution alkaline with ammonia.
Reaction takes place in the aqueous solution can be shown as:
![]()