Rate of a Chemical Reaction
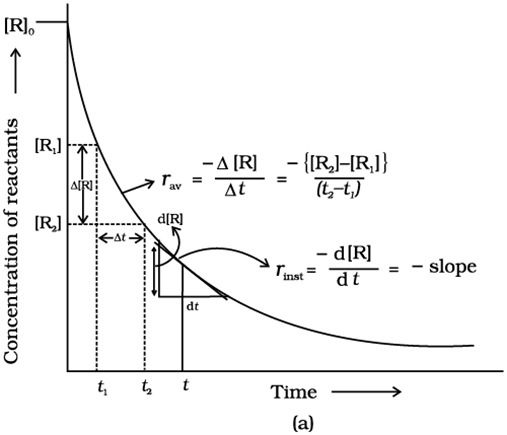
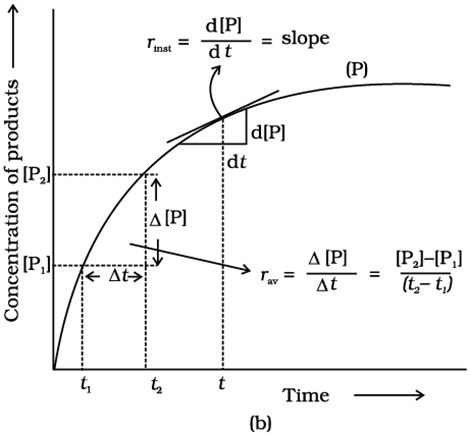
The rate of a chemical reaction is the change in the concentration of any one of the reactants or products per unit of time. It is expressed in mol L-1s-1or Ms-1or atm time-1units.
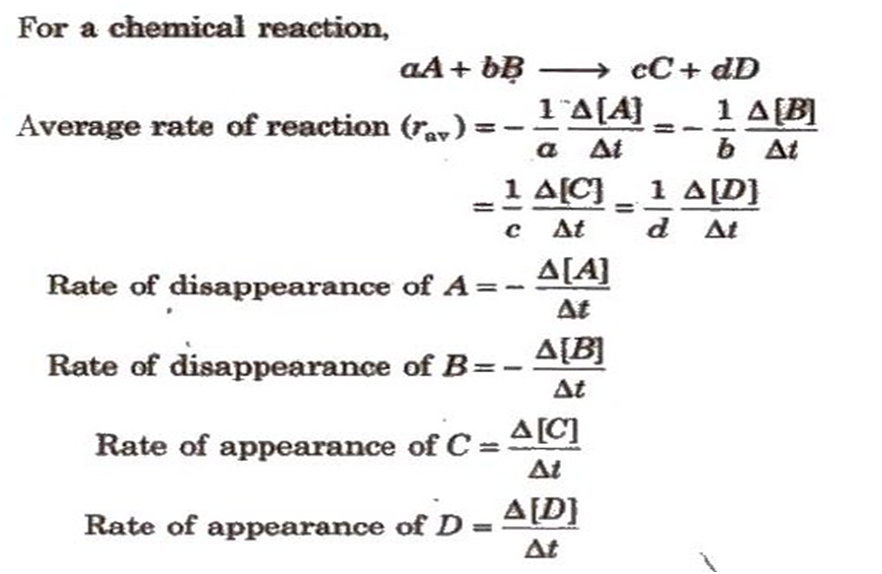
(i) the rate of decrease in concentration of any one of the reactants, or
(ii) the rate of increase in concentration of any one of the products.
R→P
One mole of the reactant R produces one mole of the product P. If [R]1and [P]1are the concentrations of R and P respectively at time t1and [R]2and [P]2are their concentrations at time t2then,
∆t=t2–t1
∆[R] = [R]2– [R]1
∆[P] = [P]2– [P]1
The square brackets in the above expressions are used to express molar concentration.
![]() (1)
(1)
![]() (2)
(2)
Since ∆[R] is a negative quantity (as the concentration of reactants is decreasing), it is multiplied by –1 to make the rate of the reaction a positive quantity.
Equations (1) and (2) given above represent theaverage rate of a reaction,rav.
From equations (1) and (2), it is clear that units of rate are concentration time–1. For example, if the concentration is in mol L–1and time is in seconds then the units will be mol L-1s–1. However, in gaseous reactions, when the concentration of gases is expressed in terms of their partial pressures, then the units of the rate equation will be atm s–1.
For a chemical reaction,
The rate of a chemical reaction at a particular moment of time is known as the instantaneous rate of reaction.
For reaction,
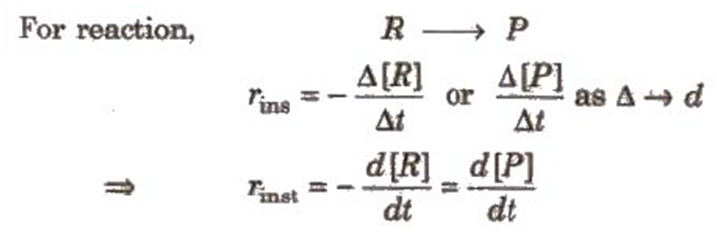
Now consider a reaction
Hg(l) + Cl2(g)→HgCl2(s)
Where stoichiometric coefficients of the reactants and products are the same, then the rate of the reaction is given as
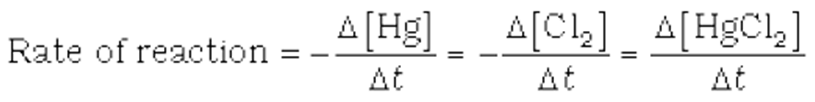
2HI(g)→H2(g) + I2(g)
For expressing the rate of such a reaction where the stoichiometric coefficients of reactants or products are not equal to one, the rate of disappearance of any of the reactants or the rate of appearance of products is divided by their respective stoichiometric coefficients. Since rate of consumption of HI is twice the rate of formation of H2or I2, to make them equal, the term∆[HI] is divided by 2. The rate of this reaction is given by
Rate of reaction ![]()
5 Br-(aq) + BrO3–(aq) + 6 H+(aq)→3 Br2(aq) + 3 H2O (l)
![]()
For a gaseous reaction at a constant temperature, concentration is directly proportional to the partial pressure of a species, and hence, the rate can also be expressed as a rate of change in the partial pressure of the reactant or the product.
Factors Influencing Rate of a Reaction
1. Nature and concentration of reactant
2. Temperature
3. Surface area of reactant
4. Radiations and catalyst
5. Pressure of gas
Dependence of Rate on Concentration
Rate Expression and Rate Constant
Rate Law Expressions
Rate Law is the expression in which reaction rate is given in terms of molar concentration of reactant with each term raised to some Power, which may or may not be same as the stoichiometric coefficient of the reacting species in a balanced chemical equation

Rate Constant
In the above expression, k is called rate constant or velocity constant
Rate constant may be defined as the specific rate of reaction when the molar concentrations of the reactants is taken to be unity, i.e.,
Rate = k, if [A] = [B] = 1
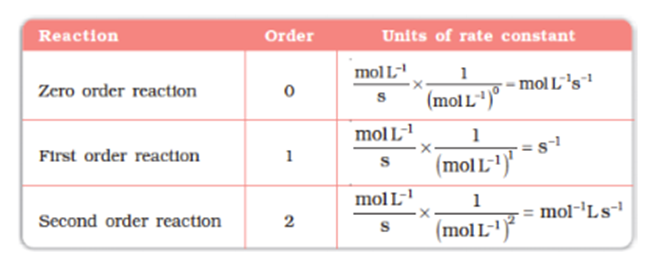
Units of rate constant or specific reaction rate for a nth order reaction is given as
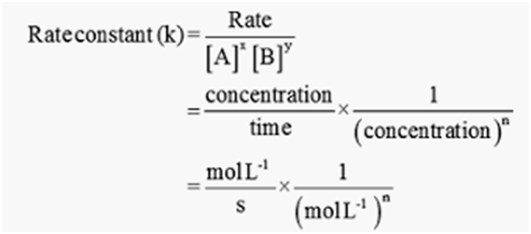
= (mol L-1)1-ns-1
Characteristics of rate constant
1 Greater the value of rate constant, faster is the reaction.
The value of rate constant for a reaction doesn’t depend upon the concentration of the reactants.
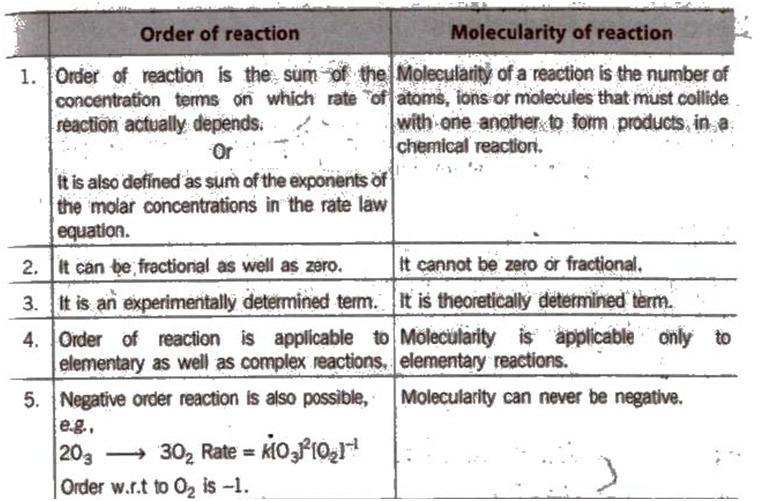
Order of a Reaction
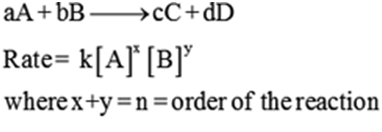
Hence, the sum of powers of the concentration of the reactants in the rate law expression is called the order of that chemical reaction.
Order of a reaction can be 0, 1, 2, 3 and even a fraction. A zero order reaction means that the rate of reaction is independent of the concentration of reactants.
A balanced chemical equation never gives us a true picture of how a reaction takes place since rarely a reaction gets completed in one step. The reactions taking place in one step are calledelementary reactions.When a sequence of elementary reactions (called mechanism) gives us the products, the reactions are calledcomplex reactions. These may be consecutive reactions (e.g., oxidation of ethane to CO2and H2O passes through a series of intermediate steps in which alcohol, aldehyde and acid are formed), reverse reactions and side reactions (e.g., nitration of phenol yieldso-nitrophenol andp-nitrophenol).
Units of rate constant
Units of rate constant
For a general reaction
aA + bB→cC + dD
Rate =k[A]x[B]y
Where x + y = n = order of the reaction
k = ![]()
![]()
Taking SI units of concentration, mol L–1and time, s, the units ofkfor different reaction order are listed in Table
Molecularity of a Reaction
Another property of a reaction called molecularity helps in understanding its mechanism.The number of reacting species (atoms, ions or molecules) taking part in an elementary reaction, which must collide simultaneously in order to bring about a chemical reaction is called themolecularityof a reaction. The reaction can be unimolecular when one reacting species is involved, for example, the decomposition of ammonium nitrite.
NH4NO2→N2+ 2H2O
Bimolecular reactions involve simultaneous collision between two species, for example, dissociation of hydrogen iodide.
Trimolecular or termolecular reactions involve simultaneous collision between three reacting species, for example,
It is, therefore, evident that complex reactions involving more than three molecules in the stoichiometric equation must take place in more than one step.
KClO3+ 6FeSO4+ 3H2SO4→KCl + 3Fe2(SO4)3+ 3H2O
This reaction which apparently seems to be of tenth order is actually a second-order reaction. This shows that this reaction takes place in several steps. Which step controls the rate of the overall reaction? The question can be answered if we go through the mechanism of reaction, for example, chances to win the relay race competition by a team depend upon the slowest person in the team. Similarly, the overall rate of the reaction is controlled by the slowest step in a reaction called the rate-determining step. Consider the decomposition of hydrogen peroxide which is catalysed by iodide ion in an alkaline medium.
2H2O2 ![]() 2H2O + O2
2H2O + O2
![]()
This reaction is first order with respect to both H2O2and I–.Evidences suggests that this reaction takes place in two steps
(1) H2O2+I–→H2O+IO–
(2) H2O2+IO–→H2O+I–+O2
Both steps are bimolecular elementary reactions. Species IO- is called an intermediate since it is formed during the course of the reaction but not in the overall balanced equation. The first step, being slow, is the rate-determining step. Thus, the rate of formation of the intermediate will determine the rate of this reaction.
Thus, from the discussion, till now, we conclude the following:
(i) The order of a reaction is an experimental quantity. It can be zero and even a fraction but molecularity cannot be zero or a non-integer.
(ii) Order is applicable to elementary as well as complex reactions whereas molecularity is applicable only for elementary reactions. For complex reactions, molecularity has no meaning.
(iii) For complex reactions, the order is given by the slowest step and the molecularity of the slowest step is the same as the order of the overall reaction.