PURIFICATION AND CHARACTERISATION OF ORGANIC COMPOUNDS
METHODS OF PURIFICATION OF ORGANIC COMPOUNDS
Need for purification:
In order to study the structure, physical properties, chemical properties and biological properties of organic compounds they must be in the pure state.
There are several methods by which organic compounds can be purified. The methods
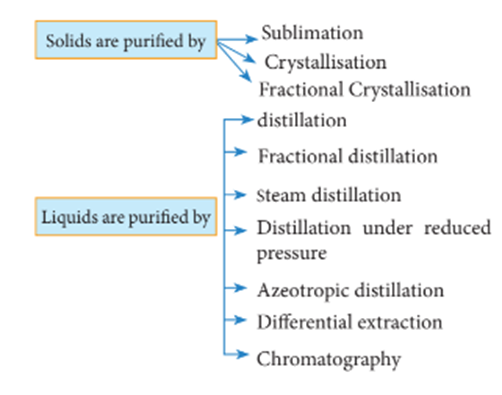
Crystallization
It is the most widely used method for the purification of solid organic compound.
(i) Selection of solvent: Most of the organic substances being covalent do not
ii) Preparation of solution: The organic substance is dissolved in the minimum amount of the selected solvent. A small amount of animal charcoal can be added to remove any colored impurities. The heating can be done over a wire gauze or water bath depending on the nature of the liquid.
iii) Filtration of hot solution: The hot solution is then filtered through a fluted filter paper placed in a funnel to remove any insoluble impurities.
iv) Crystallization: The hot filtrate is allowed to cool slowly to room temperature. As the solution cools, the pure solid substance starts to crystallize out. Most of the impurities remain in the solution or get trapped in the crystals. If the rate of crystallization is slow, it can be induced by scratching the walls of the beaker with a glass rod or by adding a few crystals of the pure compound to the solution.
v) Isolation and drying of crystals: The crystals are separated from the mother liquor by filtration. Filtration is done under reduced pressure using a Buchner funnel. The crystals are washed with small quantities of cold, pure solvent to remove any remaining impurities, and then dried in a desiccator. The final product is obtained as pure crystals of the organic substance.
Distillation
Distillation is a process of separating the components of a mixture based on their different boiling points. In this process, a liquid mixture is heated and vaporized, and the resulting vapor is then condensed and collected as a purified liquid. The boiling points of the components in the mixture determine the order in which they will vaporize and condense. The process is commonly used in chemical laboratories and in industry for the production of chemicals, fuels, and other products. Different types of distillation methods, such as simple distillation, fractional distillation, and steam distillation, are used depending on the specific requirements of the separation.
Fractional Distillation
Fractional distillation is a separation technique that is used to separate a mixture of two or more liquids with different boiling points. It is based on the fact that when a mixture of liquids is heated, the liquid with the lower boiling point will vaporize first. The vapor will then be condensed and collected as a liquid, which can be separated from the original mixture.
The process of fractional distillation involves several steps. First, the mixture is heated in a distillation flask, and the vapors are collected in a fractionating column. The column is packed with glass beads or other materials to provide a large surface area for the vapors to condense and re-vaporize. As the vapors rise up the column, they will cool and condense at different heights depending on their boiling points. The cooler liquid will then run back down into the distillation flask, while the vapor will continue to rise up the column.
The temperature of the distillation flask is gradually increased, allowing each component of the mixture to boil off and condense at different heights in the column. As each component reaches its boiling point, it will be collected in a separate flask. This process is repeated until all the components of the mixture have been collected.
The key to the success of fractional distillation is the fractionating column, which provides multiple surfaces for the vapors to condense and re-vaporize. This allows for a more efficient separation of the mixture into its individual components. Fractional distillation is commonly used in the petroleum industry to separate crude oil into its different components, such as gasoline, diesel fuel, and kerosene. It is also used in the production of alcoholic beverages and in the laboratory to purify chemicals.
Steam Distillation
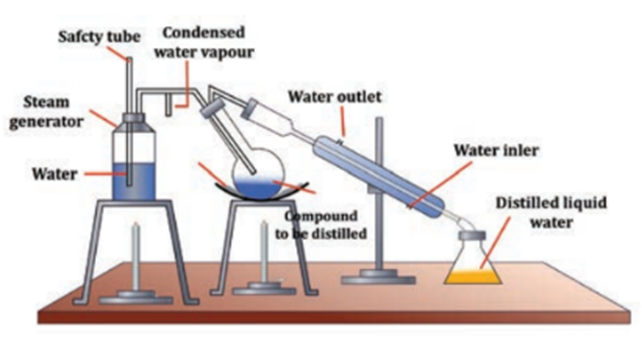
Steam distillation is a separation technique used to extract volatile organic compounds from a mixture. It is particularly useful for isolating compounds that are sensitive to high temperatures. The process involves passing steam through a mixture of an immiscible liquid and the volatile organic compound, causing the organic compound to vaporize. The mixture of steam and organic vapors is then condensed, and the two layers are separated.
The working principle of steam distillation is based on the fact that when steam is passed through a mixture of an immiscible liquid and a volatile organic compound, the vapor pressure of the mixture is lowered. This causes the organic compound to vaporize at a lower temperature than it would under normal conditions. The steam carries the vaporized organic compound with it, and the mixture of steam and organic vapors is then condensed. The condensate is collected in a receiver, where the two layers separate due to their immiscibility. The organic layer, being less dense, floats on top of the aqueous layer.
Steam distillation is commonly used in the extraction of essential oils from plants. In this process, steam is passed through the plant material, causing the essential oil to vaporize along with the steam. The steam and oil mixture is then condensed, and the oil is separated from the aqueous layer. Steam distillation is also used in the purification of organic compounds, such as amino acids and fatty acids.
One advantage of steam distillation is that it is a gentle process that does not require high temperatures, which can be harmful to some organic compounds. However, it is important to use caution when handling steam and hot water to avoid burns or scalds.
Differential Extraction
Differential extraction is a technique used in organic chemistry to separate a mixture of organic compounds based on their relative solubilities in two different immiscible solvents. It is particularly useful for separating a mixture of compounds with different polarities. The two solvents used should be immiscible, which means they don't mix together, such as water and an organic solvent like ether or chloroform.
The general working principle of differential extraction involves mixing the mixture of compounds with one of the solvents and then separating the two phases based on their different densities. The more polar compounds will typically remain in the aqueous phase, while the less polar compounds will partition into the organic phase.
The organic phase can then be separated from the aqueous phase and evaporated to obtain the less polar compounds. The aqueous phase can be further treated to isolate the more polar compounds.
Overall, differential extraction is a powerful technique for separating a mixture of compounds with different polarities and is widely used in organic chemistry for purification and isolation of compounds.
Chromatography is a separation technique used to separate the components of a mixture based on their different interactions with a stationary and a mobile phase. The mixture to be separated is dissolved in a mobile phase, which moves over the stationary phase. The stationary phase can be a solid or liquid, and the mobile phase can be a gas or liquid.
As the mixture moves over the stationary phase, the components of the mixture interact differently with the stationary phase, causing them to move at different rates. The slower the interaction, the longer the component stays on the stationary phase, and the faster the interaction, the quicker the component moves with the mobile phase.
The different types of chromatography include gas chromatography, liquid chromatography, ion exchange chromatography, and size-exclusion chromatography, among others. Each type of chromatography is based on different principles of separation, such as the differences in boiling points, solubility, charge, or size of the components.
Chromatography has a wide range of applications in various fields, such as chemistry, biochemistry, pharmaceuticals, forensics, and environmental science, among others. It is an essential tool for analyzing complex mixtures and isolating individual components for further study or use.
Adsorption chromatography
Adsorption chromatography is a type of chromatography where the stationary phase is a solid material that has adsorptive properties towards the analyte of interest. The adsorptive material is typically a polar or charged substance like silica gel, alumina, or cellulose.
The process works on the principle that the analyte will be adsorbed onto the stationary phase to varying degrees, depending on its polarity and other chemical properties. A sample is applied to the top of the column, and as it passes through, the different components of the sample interact with the stationary phase to varying degrees, causing them to separate.
As an example, consider the separation of a mixture of pigments using adsorption chromatography. Silica gel, a commonly used stationary phase, is first prepared by washing with a polar solvent, such as methanol, and then packed into a column. The mixture of pigments is dissolved in a nonpolar solvent, such as hexane, and applied to the top of the column.
As the sample passes through the column, the more polar pigments will interact more strongly with the stationary phase and be adsorbed more tightly, while the less polar pigments will pass through the column more quickly. By adjusting the polarity of the solvent used to elute the pigments from the column, they can be separated based on their relative polarities, resulting in distinct bands or spots on the column that correspond to individual pigments. These separated pigments can then be collected and further analyzed or used for other purposes.
Column chromatography
Column chromatography is a widely used technique in organic chemistry for the separation of components of a mixture based on their different interactions with a stationary phase and a mobile phase. It involves packing a glass column with a stationary phase (e.g. silica gel or alumina) and passing a mobile phase (e.g. a solvent or a mixture of solvents) through it to elute the components of the mixture.
The sample mixture is loaded onto the top of the column, and as the mobile phase is passed through the column, the components of the mixture interact differently with the stationary phase. The more strongly adsorbed components will move more slowly through the column, while the weakly adsorbed components will move more quickly.
The eluted fractions are collected in test tubes or flasks as they come off the column. The purity and identity of each fraction can be determined by analyzing them using various techniques such as thin-layer chromatography (TLC), gas chromatography (GC), or high-performance liquid chromatography (HPLC).
An example of column chromatography is the separation of a mixture of two components, A and B, where A is more polar than B. The stationary phase used in this case would be silica gel, which is polar, and the mobile phase would be a non-polar solvent such as hexane. When the mixture is passed through the column, component B will be eluted first as it interacts less strongly with the stationary phase, while component A will be eluted later as it interacts more strongly with the stationary phase.
Thin layer chromatography
Thin layer chromatography (TLC) is a type of chromatography commonly used in organic chemistry to separate and identify components of a mixture. In TLC, a thin layer of adsorbent material, such as silica gel or alumina, is coated onto a flat glass or plastic plate.
The sample to be separated is applied as a small spot near the bottom of the plate, and the plate is then placed in a developing chamber containing a solvent system. As the solvent rises up the plate by capillary action, it carries the components of the sample with it. Each component interacts differently with the stationary phase and mobile phase, causing them to travel at different rates and separate from each other.
Once the components have separated to a sufficient degree, the plate is removed from the developing chamber and allowed to dry. The separated components can then be visualized by staining the plate with a suitable reagent or by using UV light. The distance traveled by each component is measured as its Rf value, which is a ratio of the distance traveled by the component to the distance traveled by the solvent front.
TLC is widely used in organic chemistry for the analysis and purification of organic compounds, such as amino acids, sugars, and steroids. For example, a mixture of amino acids can be separated on a silica gel TLC plate using a solvent system consisting of water and an organic solvent, and the individual amino acids can be identified by their unique Rf values.
Partition chromatography
Partition chromatography is a type of liquid-liquid chromatography (LLC) that relies on the distribution of a solute between two immiscible liquid phases. The stationary phase is a liquid that is immobilized on a solid support, while the mobile phase is another liquid that is allowed to flow over the stationary phase.
In partition chromatography, the separation is based on the differential solubility of the solutes in the two liquid phases. The more soluble a solute is in the stationary phase, the slower it will move through the column, and vice versa. This differential solubility is controlled by adjusting the composition of the two liquid phases.
One example of partition chromatography is the separation of amino acids by the solvent system used by Ninhydrin. In this technique, an amino acid mixture is added to a column containing a stationary phase such as cellulose or silica gel. The column is then eluted with a solvent mixture consisting of butanol, acetic acid, and water. As the solvent flows over the stationary phase, the amino acids are partitioned between the two liquid phases based on their solubility, and they are separated accordingly. The amino acids can be detected using Ninhydrin, which reacts with the amino group to produce a colored compound.
Qualitative analysis of organic compounds
Qualitative analysis of organic compounds involves the identification of functional groups present in the given sample. The following are the steps involved in qualitative analysis:
1. Physical examination: The sample is observed for its physical properties like color, odor, melting and boiling points, and solubility in different solvents.
2. Elemental analysis: The percentage of carbon, hydrogen, nitrogen, sulfur, and halogens are determined by combustion analysis.
3. Derivatization: The sample is treated with specific reagents to form derivatives of the functional groups present. For example, the addition of 2,4-dinitrophenylhydrazine to a carbonyl compound produces a yellow-orange precipitate indicating the presence of a carbonyl group.
4. Chromatographic separation: The sample is separated using different types of chromatography, such as column chromatography, thin-layer chromatography, and gas chromatography.
5. Spectroscopic analysis: Various spectroscopic techniques like infrared spectroscopy, nuclear magnetic resonance spectroscopy, and mass spectroscopy are used to determine the functional groups present in the sample.
By combining the results obtained from these techniques, the functional groups present in the sample can be identified, and the organic compound can be characterized.
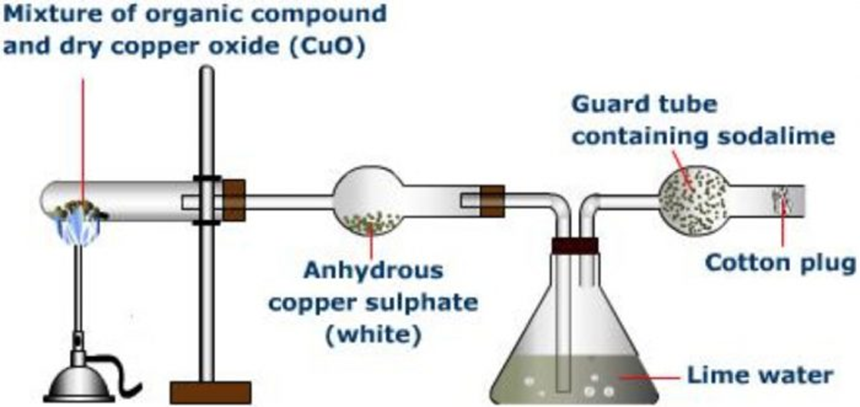
Principle:The presence of carbon and hydrogen, in an organic compound, is detected by heating thegiven compound with dryCopper(II)oxideor cupric oxide in a hard glass test tube when carbon present isoxidised to carbon dioxide and hydrogen is oxidised to water.
C +2 CuO —-> CO2+ 2 Cu
2H + CuO ———-> H2O + Cu
In general, if the organic compound containing only C and H has the molecular formula C,H, then thecomplete combustion equation may be written as
CxHy+(2 x + y/2) CuO——–> xCO2+ y/2 H2O+ (2x + y/2)Cu
Carbon dioxide turns lime water milky while water condenses on the cooler parts of the test tube andturns anhydrous copper sulphate blue.
Ca(OH)2+ CO2——-> CaCO3+ H2O
CuSO4+ 5 H2O ———> CuSO4.5H2O
Modifications in the estimation of Carbon and Hydrogen
(1) If the organic substance is a volatile liquid or a gas, the vapours of the compound are passed through heated cupric oxide taken in a hard glass test tube and gases evolved are tested for CO2and H2O vapours.
(2) If the organic compound also contains sulphur then itis oxidised to sulphur dioxide which also turns lime water milky due to the formation of insoluble calcium sulphite.
4CuO + S ——-> 2 Cu2O + SO2
Ca(OH)2+ SO2———> CaSO3+ H2O
The out coming gases are first passed through an acidified solution of potassium dichromate which absorbs sulphur dioxide and turns it green, and then through lime water.
Detection of Other Elements
Test for Nitrogen
The presence of nitrogen is detected by the following tests:
1. Dry heating test.If the organic compound containing nitrogen is heated strongly, it gives a smell of burning hair.
Limitation.This test is, however, not reliable since many compounds containing nitrogen do not give this test.
2. Soda lime test.A pinch of the organic compound is heated strongly in a dry test tube withsoda-lime(CaO+ NaOH).A smell of ammonia indicates the presence of nitrogen.
NH2CONH2+ 2 NaOH ——–> 2NH3+ Na2CO3
Limitation.This test is also not reliable since many organic compounds containing nitrogen such nitro , azo groups, etc. do not give this test.
3. Lassaigne’s test.In this test, the elements present in the organic compound are converted from covalent form into the ionic form by fusing the compound with sodium metal. This test is carried out as follows:
(1) Preparation of the Lassaigne’s extract.A small piece of freshly cut sodium is heated gently in a fusion tube till it forms a shiny globule.The tube is removed from the flame and a small amount of the organic compound (50-60 mg) is added and the tube heated strongly till it becomes red hot(2–3 minutes). The hot tube is then plunged into a china dish containing 10 – 15 ml. of distilled water. The contents of the china dish are boiled for a few minutes, cooled and then filtered. The filtrate is calledLassaigne’s extract or sodium fusion extract.
(a) Test for Nitrogen.The Lassaigne’s extract is alkaline since the excess of sodium react with water to produce sodium hydroxide. If not, it is made alkaline by adding a few drops of a dilute solution of sodium hydroxide. To a part of this alkaline solution is added a few drops of a freshly prepared solution of ferrous sulphate. The contents are warmed a little, cooled and then acidified with dil H2SO4.
Appearance of a green or blue colouration indicates the presence ofnitrogen. However, appearance of blood colour indicates the presence of both Nitrogen and sulphur.
Chemistry of the test.During fusion, carbon and nitrogen of the organic compound combine to form sodium cyanide.
Na + C + N ———-> NaCN
On heating with ferrous sulphate solution, sodium ferrocyanide. i.e.,sodium hexacyanoferrate(II) is formed and at the same time some ferrous (Fe2+) ions are oxidised to ferric (Fe3+)ions. TheseFe3+ions then react then react with sodium hexacyanoferrate to produce iron (III) hexacyanoferrate which is prussion blue in colour.
2NaCN + FeSO4———> Na2SO4+ Fe(CN)2
Fe(CN)2+ 4 NaCN ——-> Na4[Fe(CN)6]
3Na4[Fe(CN)6] +4 Fe3+———-> Fe4[Fe(CN)6]3+ 12 Na+
If nitrogen and sulphur both are present in the organic compound, they may combine during fusion toform sodium thiocyanate (sulphocyanide) due to insufficient sodium. This when heated with ferrous sulphate a blood red colouration due to ferric thiocyanate (or sulphocyanide) by reaction with ferric ions isformed by oxidation of ferrous ions.
Na + C + S + N —–> NaSCN
Fe3++ 3 NaSCN———-> Fe(SCN)3+ 3Na+
But the absence of blood red colouration does not necessarily mean that sulphur is absent. This is dueto the reason that in presence of excess of sodium metal, sodium thiocyanate decomposes to form sodiumcyanide and sodium sulphide
2Na + NaSCN ——-> Na2S+ NaCN
Test for Sulphur
The sulphur in the organic compound is detected by the following tests:
(1) Lassaigne’s test
If sulphur is present in the organic compound then on fusion with sodium metal , sodium sulphide is formed.
2 Na + S —->Na2S
(a)Sodium nitroprusside test :
A small portion of the Lassaigne’s filtrate is treated with a few drops of sodium nitroprusside solution when a violet colouration is obtained. This colour slowly fades on standing.
Na2S + Na2[Fe(CN)5(NO)] ———> Na4[Fe(CN)5(NOS)]
(b) Lead acetate test.
Another portion of Lassaigne’s filtrate is acidified with dilute acetic acid and afew drops or lead acetate solution are added to it. Formation of black lead acetatethe presence of sulphur in the given compound.
Na2S + (CH3COOH)2Pb ———> PbS + 2CH3COONa
(c) Oxidation test
The given compound is fused with a mixture of potassium nitrate and sodium carbonate. If sulphur is present, it gets oxidised to sulphate.
KNO3——–> KNO2+ [O]
Na2CO3+ S + 3[O] ——-> Na2SO4+ CO2
given compound is fused with a mixture of potassium nitrate and sodiumThe fused mass is then extracted with water and filtered. The filtrate is first acidified with dilute
hydrochloric acid and then treated with barium chloride solution when a white precipitate insoluble inhydrochloric acid is obtained.
Na2SO4+ BaCl2———> 2NaCl + BaSO4
Test for Halogens
The presence of halogens in an organic compound is detected by the following tests:
(1) Beilstein test:In this test, a clean and stout copper wire is heated in the non-luminous flame of the Bunsenburner until it ceases to impart any green or bluish green colour to the flame. The heated end is then dipped in the organic compound and again introduced into the Bunsen flame. The appearance of a green or bluish flame due to theformation of volatile cupric halides indicates the presence of halogens in the organic compounds.
Limitations
(1) Organic compounds like urea, thiourea, etc. which do not contain halogens also give this due to the formation ofvolatile cupric cyanide.
(2) It not tell as to which halogen (chlorine, bromine, or iodine) is actually present in the organic compound.
(2) Lassaigne’s test.It is a very reliable test for the detection of halogens in an organic compound.
Preparation of the Lassaigne’s extract
(1) Test for halogens.A part of the Lassaigne’s extract is boiled with dil. HNO3and cooled. A fewdrops of silver nitrate solution are then added.
(a)A white precipitatesoluble in ammonia and insoluble in dil. HNO, indicates the presence of chlorine.
NaCl +AgNO3———> AgCl+ NaNO3
(b)A pale yellow precipitate partially soluble in ammonia indicates the presence of bromine.
NaNO3+ AgBr ———> AgBr + NaNO3
c)A yellow precipitate insoluble in ammonia indicates the presence of iodine.
NaI + AgNO3——–> AgI +NaNO3
Function of Nitric Acid
If the organic compound also contains nitrogen or sulphur, the Lassaigne’s extract on boiling with dil. HNO3decomposes sodium cyanide or sodium sulphide formed during fusion.
If cyanide and sulphide ions are not decomposed, they will react with silver nitrate and hence will interfere with the test.
NaCN+AgNO3——-> AgCN+ NaNO3
Na2S + 2 AgNO3——> Ag2S + 2 NaNO3
(3) Carbon disulphide test for Bromine and Iodine
A small portion of the Lassaigne’s extract is boiled with dil H2SO4, to decompose sodium cyanide and sodium sulphide. The solution is then cooled and a few mL of freshly prepared chlorine water and carbon disulphide or carbon tetrachloride are added. The solution is vigorously shaken and allowed to stand when:
(1) An orange colour in CS2or CCl4layer indicates the presence of bromine.
(2) A violet colour in CS2or CCl4, layer indicates the presence of iodine.
Theory of the test.Chlorine displaces bromine and iodine from their corresponding halides. The halogen thus liberated dissolves in CS2or CCl4, to produce the specific colour.
2 NaBr +Cl2——–>2NaCl +Br2(Dissolves in CS2 or CCl4 to give orange colour)
2NaI + Cl2——-> 2NaCl+ I2(Dissolves in CS2, or CCl4, to give violet colour)
Test for Phosphorus
Phosphorus is detected by fusing the organic compound with an oxidising agent, i.e., sodium peroxide when phosphorusis oxidised to sodium phosphate.
5 Na2O2+ 2 P ——> 2 Na3PO4+ 2 Na2O
The fused mass is extracted with water. The aqueous solution is boiled with conc HNO3, and then ammonium molybdate solution is added. The appearance of a yellow precipitate or colouration due to theformation of ammonium phosphomolybdate indicates the presence of phosphorus.
Na3PO4+ 3HNO3——> H3PO4+ NaNO3
H3PO4+12 (NH4) MoO4+21 HNO3——-> (NH4)3PO4. 12MoO3+21 NH4NO3+ 12H2O
Quantitative analysis of organic compounds
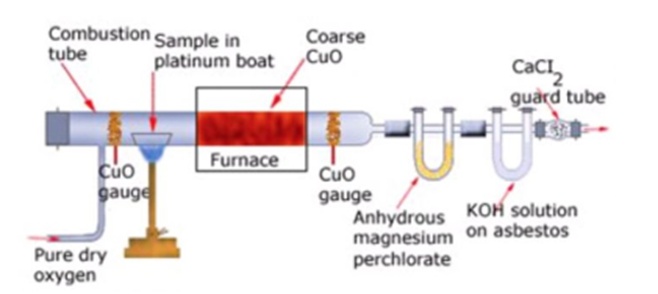
Quantitative analysis of Carbon and Hydrogen
Principle. A known mass of the organic compoundis heated strongly with excess of dry copper oxidein a current of dry air or oxygen (free from carbon dioxide). Under these conditions, carbon present in theorganic compound is oxidized to carbon dioxide and hydrogen is oxidized to water.
C (from organic compound) + 2CuO——->CO2+ 2 Cu
2H (from organic compound) + CuO———>H2O+Cu
The water thus produced is absorbed in a U-tube containing anhydrous calcium chloride or anhydrousmagnesium perchlorate while CO2produced is absorbed in another U-tube containing a strong solution ofKOH or ascarite (NaOH+ CaO). The tubes are weighed before and after the combustion. The increase inthe mass of CaCl2or Mg(ClO4)2U-tube gives the mass of water produced while increase in the mass ofKOH or ascarite U-tube gives the mass of CO2produced.
Theapparatus used for estimation of carbon and hydrogen
Calculations: Let the mass of the substance taken =w g
Mass of CO, formed = x g
Mass of water formed = y g
Percentage of Carbon
One mole of CO2contains one gram atom of C
i.e. (12 + 2×16 ) = 44 g CO2contain carbon= 12g
∴ x g CO2will contain carbon = (12/44)×× g
This is the mass of carbon present in w g of the compound
%age of carbon in the compound = (12/44)××× (100/w)
Percentage of carbon =(12/44) × (Mass of CO2formed/Mass of substance taken)× 100
Percentage of Hydrogen
One mole of H20 contains 2 gram atoms of hydrogen
ie. (2×1 + 16)= 18 g of H20 contain hydrogen= 2 g
∴ y g H20 will contain hydrogen = 2/18×y g
This is the mass of hydrogen present in w g of the compound.
% age of hydrogen in the compound =(2/18)×y×(100/w)
Percentage of hydrogen = (2/18 )×(Mass of H2O formed / mass of substance taken )×100
Modifications under specific conditions.
Liebeg’s method is suitable in case oforganic compounds containing C. H and O only. If, however, the organic compound contains one or moreof the elements like N. S and halogens also, the method is modified as under
(1)Substances containing nitrogen: Under the conditions of combustion, nitrogen, if present, in theorganic compound is oxidised to oxides of nitrogen (NO, NO2) which are also absorbed in KOHsolution. In such cases, a reduced copper gauze is placed near the exit end of the tube whichreduces oxides of nitrogen back to N2gas
2Cu + 2 NO ———> 2 CuO + N2
4Cu + 2 NO2——> 4 CuO + N2
(2) Substances containing halogens: Halogens, if present, in the organic compound are convertedinto cupric halides which themselves decompose to form free halogens. These halogens and volatile cuprichalides also get dissolved in KOH solution. In such cases, a roll of bright silver gauze is placed near the exitend of the combustion tube. Silver gauze decomposes, volatile cupric halides forming non-volatile silverhalides. Halogens also combine with silver giving silver halides.
CuX2+ 2 Ag —–> 2 AgX + Cu
4Cu + 2 NO2——–>4 CuO + N2
(3) Substances containing sulphur or sulphur and halogens:
Sulphur if present in the organic compound is oxidised to SO2which will also be absorbed in KOH solution.Insuch cases, either an additional layer of fused lead chromate is placed after the CuO layer or CuO layeris replaced by fused lead chromate layer. At high temperatures, lead chromate decomposes to give O2whichoxidises C and H of the organic compound to CO2and H20 respectively.
PbCrO4——–> 4 PbO + 2 Cr2O3+ 3 O2
4PbCrO4+ SO2———> 4 PbSO4+ 2Cr2O3+ 3 O2
4PbCrO4+ 4 X2—–> 4 PbX2+2Cr2O3+ 3 O2
Besides, S and halogens, PbCrO4, is also useful for phosphorus containing compounds. This is because oxides of phosphorus produced during combustion also react with lead chromate to form non-volatile lead phosphate.
Quantitative analysis of Nitrogen
The two most commonly used methods for the estimation of nitrogen in an organic compound are:
(1) Dumas method
(2) Kjeldahl’s method
Dumas method
This method is applicable to all organic compounds containing nitrogen.
Principle:A known mass of the organic substance is heated withexcess of copper oxide in anatmosphere of CO2. Carbon, hydrogen and sulphur (f present) are oxidised to CO2, H2O and SO2respectivelywhile nitrogen gas is set free. Any oxide of nitrogen that may be formed is reduced back to free nitrogen by passingover a hot reduced copper gauze.
C + 2 CuO —-> CO2+ 2 Cu
2H + CuO ——-> H2O + Cu
Nitrogen + CuO ——-> N2+ a small amount of oxides of nitrogen
Oxides of nitrogen + Cu ——–> CuO + N2
The nitrogen thus formed is collected over conc. KOH solution which absorbs all other gases i.e.CO2, H2O vapours, SO2etc. The volume of nitrogen collected is thus noted and from this the percentage ofnitrogen can be calculated.
Apparatus. The apparatus used for estimation of nitrogen by Duma’s method is
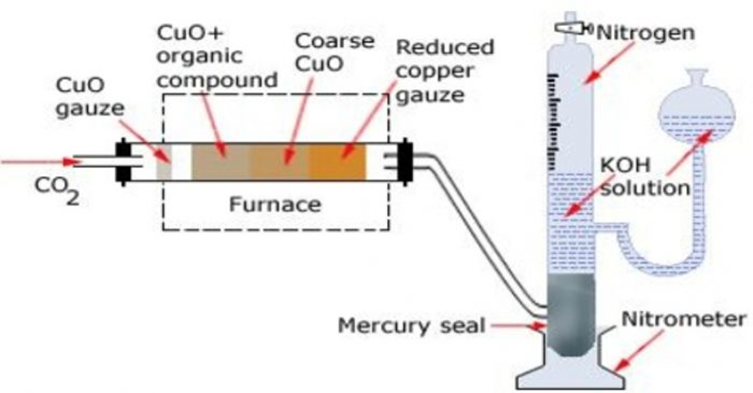
It consists of three parts :
(a) carbon dioxide generator
(b) combustion tube and
(c) Schiff’s nitrometer.
Carbon dioxide generator
CO2needed for the purpose is producedby heating sodium bicarbonate or magnesium carbonate.The gas is perfectly dried by bubbling through conc H2SO4before passing it through the combustion tube.
Combustion tube
It is a hard glass test tube about 90 cm long and about 2 cm in diameter. It ispacked with
(1) A roll of oxidised copper gauze which prevents backward diffusion of gases produced
during combustion
(2) An accurately weighed quantity of the substance mixed with excess of cupric oxide.
(3) Coarse CuO that fills nearly half of the combustion tube and
(4) A reduced copper gauze which helps toreduce any oxides of nitrogen formed during combustion back to nitrogen gas.
It consists of a long graduated tube having a reservoir and a tap at the upperend. Itcontains about 40% KOH solution. It also has a mercury seal at the bottom which prevent KOHsolution from being sucked back into the combustion tube.
Both CO2and H20 produced during combustion are absorbed by KOH solution white N2, is collected over it.Thevolume of N2is measured after careful levelling (by making the level of KOH in the nitrometertube and reservoir the same)
Kjeldahl’s method
It is largely used for the estimation of nitrogen in fertilizers, food stuffs, drugs, etc. The method ishowever, not applicable to compounds containing nitrogen in the ring e.g.pyridine, quinoline, etc.) andcontaining nitrogen directly linked to oxygen atom (e.g NO,) or another nitrogen atom ie, azocompounds.
The apparatus used for the estimation of nitrogen by Kjeldahl’s method.
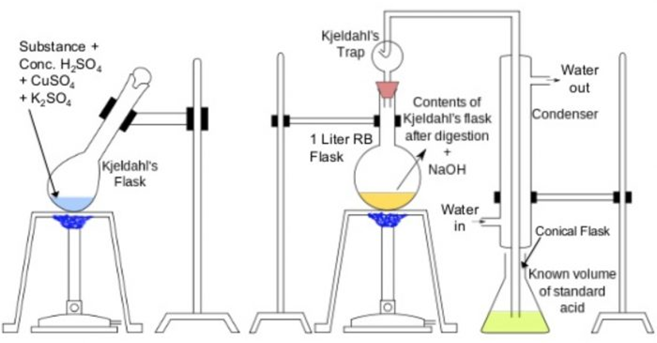
Principle: A known mass of the organic compound is heated with conc. H2SO4, in presence
of potassium sulphate and a little copper sulphate or mercury in a long-necked flask calledKjeldahl’s flask.Potassium sulphate raises the boiling point of H2SO4, and thus ensures complete reactionwhile copper sulphate or mercury catalyses the reaction.
As a result of heating, the nitrogen present in the organic compound is quantitatively converted intoammonium sulphate. Ammonium sulphate thus obtained is boiled with excess of NaOH solutionto liberate ammonia gas which is then absorbed in a known excess of a standard acid such asH2SO4, or HCl.
N (from compound) + Conc. H2SO4——–>(NH4)2SO4
(NH4)2SO4+ 2 NaOH ——–> Na2SO4+ 2 H2O + 2 NH3
2 NH3+ H2SO4———> (NH4)2SO4
The volume of the acid left unused is found by titration against a standard alkali solution.
2NaOH + H2SO4——->Na2SO4+2H2O
From this, the volume of the acid used up (or the volume of ammonia evolved) and hence the percentageof nitrogen in the organic compound can be calculated.
Calculation

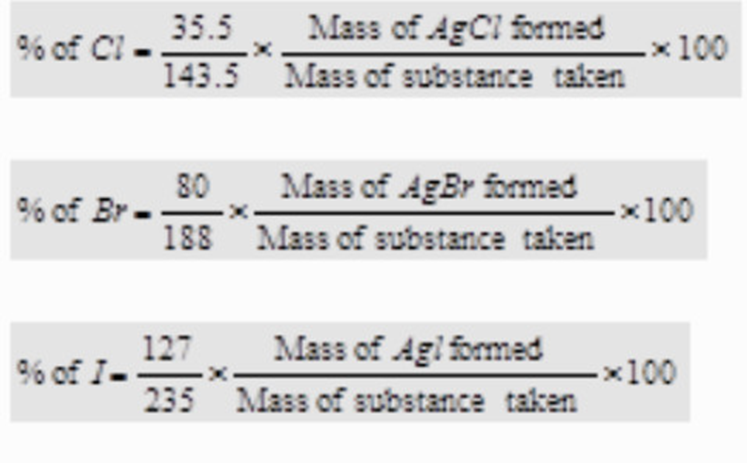
Quantitative analysis of Halogens
Carius method
Principle:A known mass of the organicsubstance is heated with fuming nitric acidand a few crystals of silver nitrate in a sealedhard glass tube (about 2 cm wide 50 cm long)calledCarius tubein a furnace.
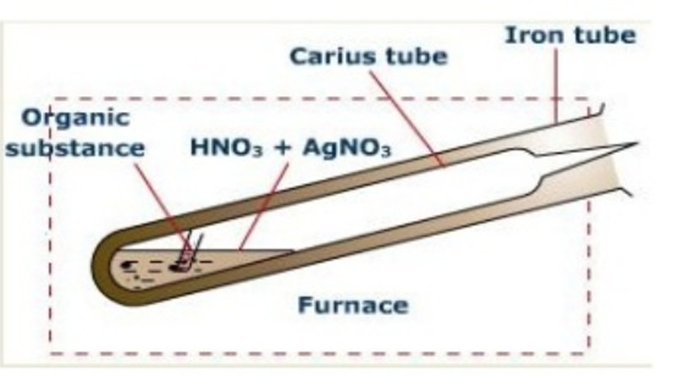
Carbon and Hydrogen are oxidised to Carbon dioxide andwater respectively while halogen is converted intosilverhalide. The precipitate of silver halide are filtered , washed ,dried and weighed.Knowing the mass of the substancetaken and the mass of the precipitate formed,
the percentage of halogen is calculated as:
Calculations:
Let the mass of the substance taken=w g
and the mass of the silver halide (AgX) formed = x g
Now, 1 mole of AgX contains 1 gram atom of X ( X= Cl, Br or I)
i.e. (108 + At. mass of X) g of AgX contain X = (At. mass of X)g
∴ x g of AgX contain X =( At.mass of X×× g )/ 108 + Atomic mass of X
%age of halogen in the compound =( At.mass of X×x g×100 ) / ((108 + Atomic mass of X))× x
Quantitative analysis of Sulphur
Principle:A known mass of the substance is heated with sodium peroxide or fuming nitric acid in asealed tube (Carius tube). Carbon and hydrogen are oxidised to CO2and H2O respectively while sulphurpresent in the compound is oxidised to sulphuric acid which is then precipitated as barium sulphate byadding excess of barium chloride solution.
C + 2O (from HNO3)——-> CO2
2H+ O (from HNO3)——-H2O
S + H2O+3O (from HNO3)——-> H2SO4
H2SO4+ BaCl2———> BaSO4+ 2HCl
The ppt. of BaSO4is filtered, washed, dried andweighed. Knowing the mass of substance takenand the mass of BaSO4ppt. formed, the percentage of sulphur can be calculated.
Calculations:
Let the mass of the substance taken w g
Mass of BaSO4ppt. formed= x g
Now, 1 mole of BaSO4contains 1 gram atom of S
i.e., (137 +32+ 64) 233 g of BaSO4, contain sulphur32 g
x g of BaSO4will contain sulphur = (32×x g )/233
This is the mass of sulphur present in w g of the substance
% age of sulphur present in the compound= (32×××100 ) / (233×w)
%age of sulphur = (32×Mass of BaSO4 formed×100)/ (233×Mass of substance taken)
Quantitative analysis of Phosphorus
Principle:A known mass of the organic compound is heated with fuming nitric acid in a sealed tube (Carius tube). Under these conditions, carbon and hydrogen are oxidised to CO2and H2O respectively while phosphoruspresent in the organic compound is oxidised to phosphoric acid which isprecipitated asammonium phosphomolybdate by heating it with conc. HNO3and then adding ammonium molybdate.
C + 2O (from HNO3)——-> CO2
2H+ O (from HNO3)——-H2O
2P + 5O (from HNO3) ——–> P2O5
P2O5+ 3 H2O ——> 2H3PO4
H3PO4+ 12 (NH4)2MoO4+ 21 HNO3——-> (NH4)3PO4.12MoO3+ 21 NH4NO3+ 12 H2O
The precipitates of amm. phosphomolybdate thus formed are filtered, washed, dried and weighed.
Alternatively phosphoric acid is precipitated as magnesium ammonium phosphate by adding magnesiamixture (a solution containing magnesium chloride, ammonium chloride and ammonia).
Mgcl2+ NH4Cl + H3PO4—–> MgNH4PO4+ 3 HCl
The precipitates of magnesium ammonium phosphate are filtered, washed, dried and then ignited.
2 MgNH4PO4——-> Mg2P2O7+ 2 NH3+ H2O
The magnesium pyrophosphate thus formed is weighed. Knowing the mass of the organic compoundtaken and the mass of ammonium phosphomolybdate or magnesium pyrophosphate formed, the percentageof phosphorus can be easily calculated.
Calculation
Mass of ammonium phosphomolybdate formed = x g
Now 1 mole of (NH4)3PO4 .12 MoO3 contains one gram atom of P i.e.
3 x (14 + 4) + 31 + 4 x 16 + 12 (96* + 3 x 16) = 1877 g of (NH4)3PO4. 12 MO3contain P = 31 g
Therefore, x g of (NH4)3PO4. 12 MO3will contain P = (31Xx)/1877 g
This is the mas of P present in w g of the compound
Therefore, % age of P = 31/1877Xx/wX100or
%age of P = 31/1877XMass of amm. phosphomolybdate /mass of substance takenX100
Alternatively if phosphorus is estimated as Mg2P2O7.
Mass of Mg2P2O7formed = x g
Now, 1 mole of Mg2P2O7formed = x g
Now, 1 mole ofMg2P2O7contains two gram atoms of P
i.e (24 X 2 + 31 X 2 + 16 X 7) = 222 g of Mg2P2O7contain phosphorus = 62 g
Therefore x g ofMg2P2O7will contain phosphorus = 62 x /222 g
This is the mass of phosphorus present in w g of the compound.
i.e. percentage of phosphorus present in the compound = (62×X×100)/ (222×w)
Therefore percentage of phosphorus =(62×Mass ofMg2P2O7formed×100)/ (222×Mass of substance taken)
The percentage of oxygen in an organic compound is usually calculated by difference method. The percentage of all other elements present in the organic compound are added and the sum is subtracted by 100.
Percentage of oxygen = 100- (Sum of the percentage of all other elements)