KETONE
Ketones are a class of organic compounds that contain a carbonyl group (C=O) bonded to two carbon atoms. The carbonyl group in a ketone is located in the middle of a carbon chain, distinguishing it from aldehydes, which have the carbonyl group at the end of a carbon chain. Ketones have the general formula R(C=O)R', where R and R' can be any alkyl, aryl, or other organic group.
Ketones are important compounds in organic chemistry and are widely used as solvents, fuels, and starting materials for the synthesis of many other organic compounds. Some common examples of ketones include acetone (the simplest ketone, also known as propanone), mesityl oxide, and cyclohexanone.
Ketones have higher boiling points than alkanes but lower boiling points than alcohols of similar molecular weight. They are polar compounds due to the presence of the carbonyl group, but they cannot form hydrogen bonds with water molecules because they do not have an OH group like alcohols. As a result, some ketones are soluble in water, but many are not.
Preparation of Ketones
Ketone from acyl chlorides
Treatment of acyl chlorides with dialkylcadmium, prepared by the reaction of cadmium chloride with Grignard reagent, gives ketones.
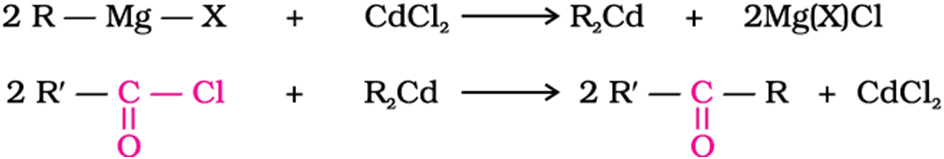
Ketone from nitriles
Treating a nitrile with Grignard reagent followed by hydrolysis yields a ketone.
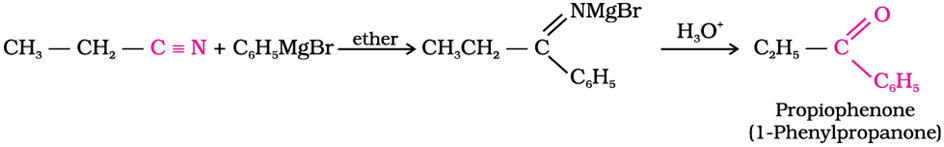
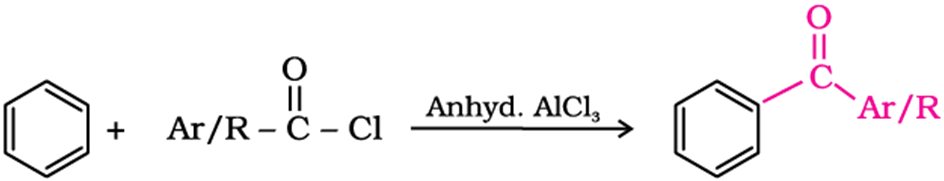
Ketone from benzene or substituted benzenes
When benzene or substituted benzene is treated with acid chloride in the presence of anhydrous aluminium chloride, it affords the corresponding ketone. This reaction is known asFriedel-Craftsacylation reaction.
Chemical reactions of ketone
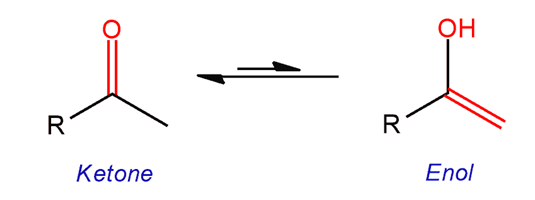
Keto-Enol Tautomerization
Ketones and other carbonyl compounds containing hydrogen adjacent to the carbonyl are inequilibriumwith a constitutionalisomer calledenol.
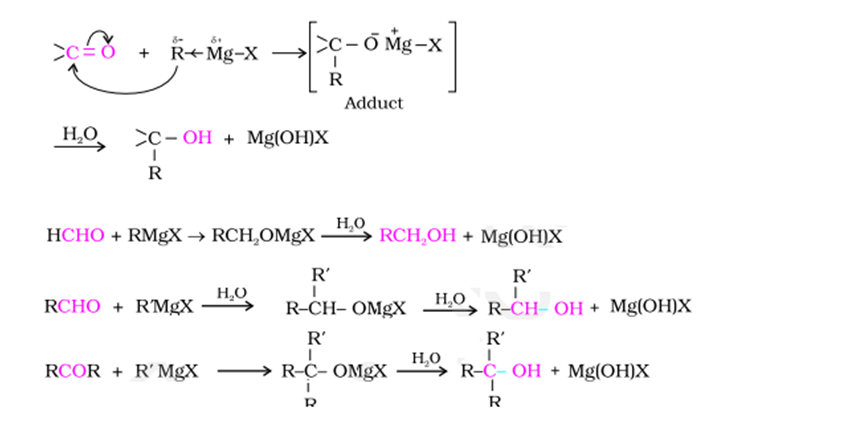
Grignard reaction mechanism
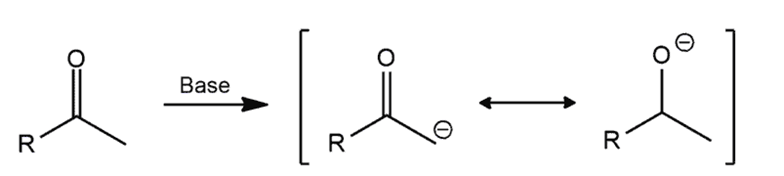
Formation of the enolate ion
ketones will react with strong base [e.g. HO(-) ] to give anenolateion.
reduction reactions of carbonyl
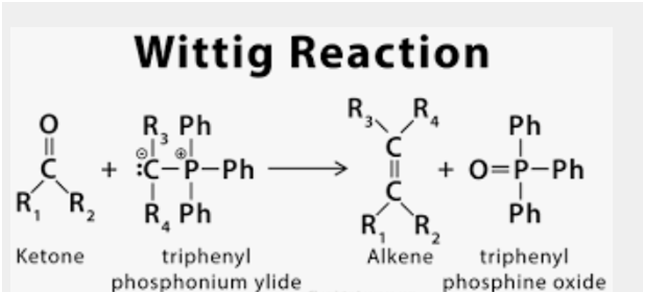
Acetal Ketal Hemiacetal Hemiketal Reaction
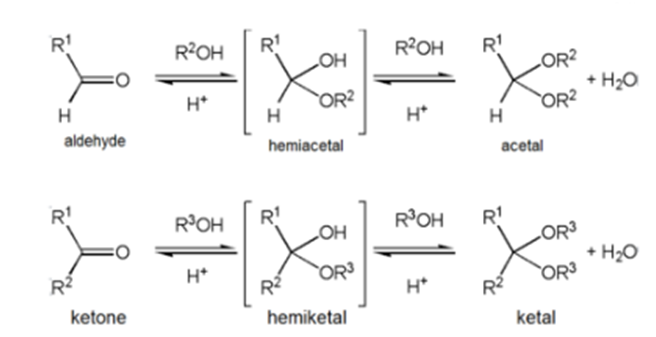
Grignard Attacks Nitrile to Form Ketone
Grignard reagents can attack the electophillic carbon in a nitrile to form an imine salt.This salt can then be hydrolyzed to become a ketone.
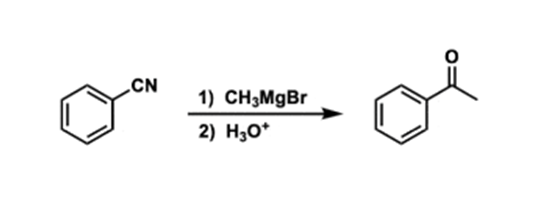
Alpha Halogenation of Ketones Reaction
Ketones and aldehydes react with halogens at the alpha position when an acid or a base catalyst is used. The halogenation works with Cl2, Br2, and I2:
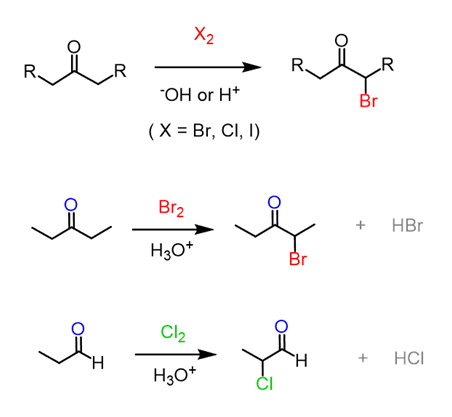
Wittig reaction
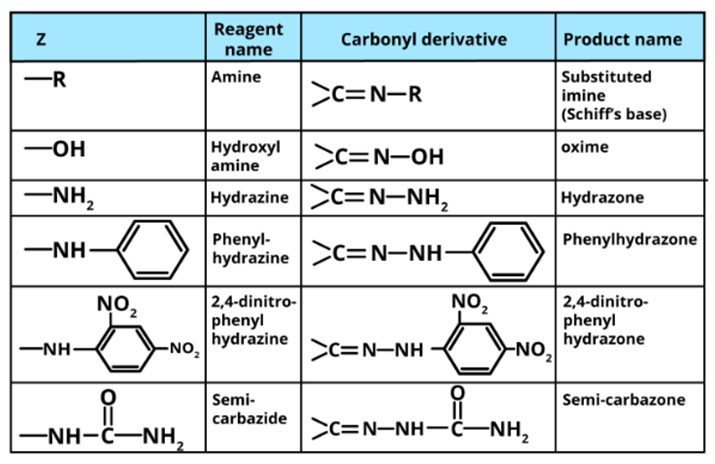
Addition of ammonia and its derivatives to ketone
Unsaturated compounds include alkenes, aldehydes, and ketones. Unlike alkenes, which undergo electrophilic addition reactions, aldehydes and ketones undergo nucleophilic addition reactions with ammonia and ammonia derivatives.
The carbonyl group is extremely polar. Since oxygen has a higher electronegativity than carbon, the carbon atom in the carbonyl group carries a small positive charge and thus behaves as an electrophile. As a result, a nucleophile can easily attack the electrophilic carbon atom of the polar carbonyl group from a direction that is perpendicular to the plane of the carbonyl carbon sp2 hybridised orbitals.
During this process, the electrons of the carbon-oxygen double bond are completely transferred from carbon to oxygen atom, the carbon hybridization changes sp2 to sp3 and a tetrahedral alkoxide intermediate is formed. This intermediate then absorbs a proton from the solvent (usually H2O) or the reagent to produce the electrically neutral addition product. The entire process involves adding Nu and H across the carbon-oxygen double bond.
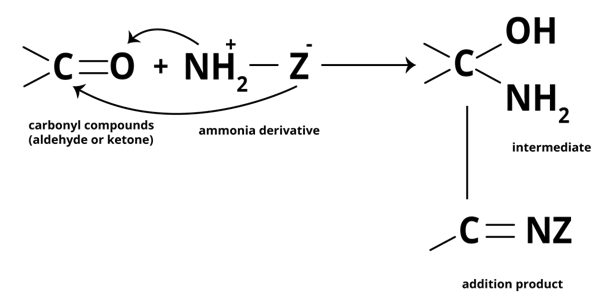
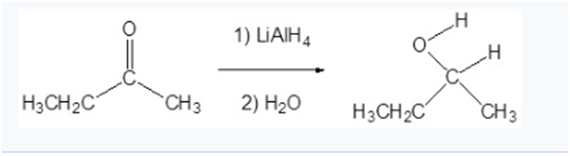
Reduction of ketone to alcohol
Addition oflithium aluminum hydridetoketonesleads to the formation ofsecondaryalcohols(after addition ofacid).
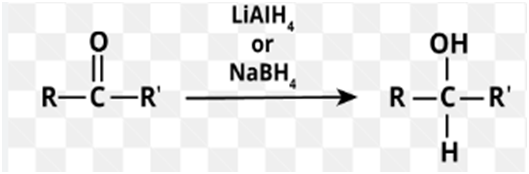
Reduction, in organic chemistry, implies a process the removal of electronegative atoms such as halogen or oxygen from the molecule, or the addition of hydrogen to the molecule. And this conversation of a ketone to asecondaryalcoholis areduction. This reaction requires areducing agent, which is itself oxidized as a result of the reaction. Such asreducing agentcan be usedlithium aluminum hydride,abbreviatedLAH, LiAlH4. Because this reagent is a source of hydride ion it can be calledhydride reagent. This is a strongnucleophilewhich attacks polarized double carbon-oxygen bond by transferringhydride ion.
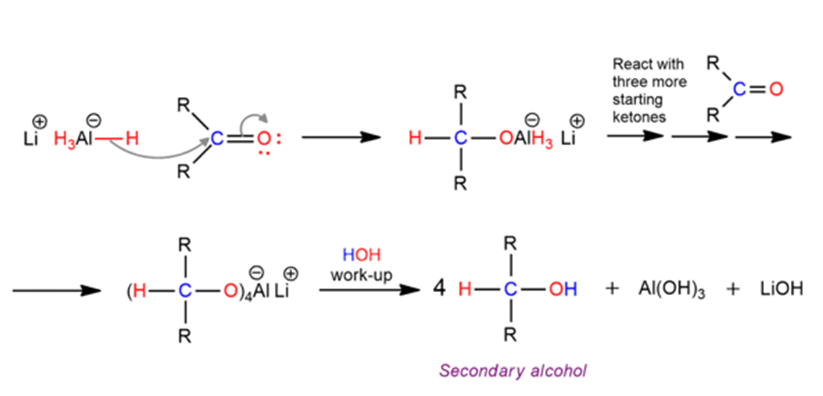
Lithium aluminum hydrideismore reactivethansodium borohydride, so their hydrogen atoms are more basic. They are attacked vigorously by water oralcoholto givehydrogen gas. Because this is not desirable, reductions are carried out inaprotic solventssuch as diethylether.
Reduction of ketone to hydrocarbons
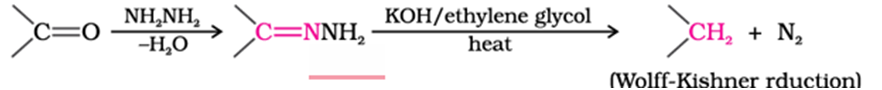
ketones is reduced to CH2group on treatment with zinc-amalgam and concentrated hydrochloric acid [Clemmensen reduction] or with hydrazine followed by heating with sodium or potassium hydroxide in high boiling solvent such as ethylene glycol (Wolff-Kishner reduction).
Clemmensen reduction of ketone
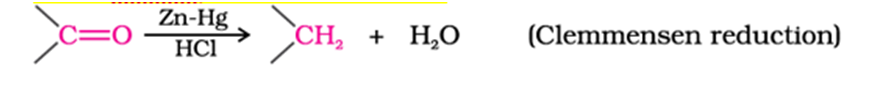
Wolff-Kishner reduction of ketone
Oxidation of ketone
Ketones are generally oxidized under vigorous conditions, i.e.,strong oxidizing agents and at elevated temperatures. The oxidation of ketonesinvolves carbon-carbon bond cleavage resulting in a mixture of carboxylic acids having lesser number of carbon atoms than the parent ketone.
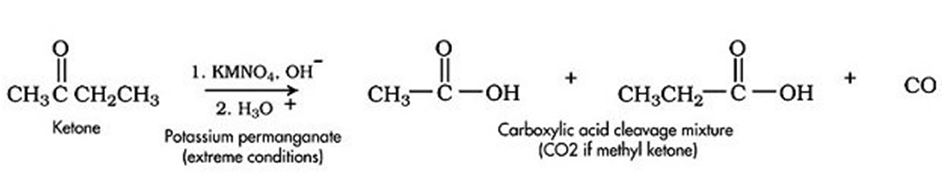
1. Ketones are not generally oxidized, even by strong oxidizing agents.
2. At high temperature, ketones are cleavage oxidized by a strong oxidizing agent like KMnO4giving carboxylic acids with number of carbon atoms less than that present in parent carbon chain. An exception is a benzylic carbonyl group, which KMnO4oxidizes easily.
3. They can also beoxidized by peracids to give esters (Baeyer Villiger Oxidation).
Tollens’ test of ketone
The Tollens' test isa reaction that is used to distinguish aldehydes from ketones, as aldehydes are able to be oxidized into a carboxylic acid while ketones cannot. Tollens' reagent, which is a mixture of silver nitrate and ammonia, oxidizes the aldehyde to a carboxylic acid.
Fehling’s test of ketone
Fehling's solution can be used to distinguish aldehyde vs ketone functional groups. The compound to be tested is added to the Fehling's solution and the mixture is heated. Aldehydes are oxidized, giving a positive result, but ketones do not react, unless they are α-hydroxy ketones.
Oxidation of methyl ketones by haloform reaction
ketones having at least one methyl group linked to the carbonyl carbon atom (methyl ketones)are oxidised by sodium hypohalite to sodium salts of corresponding carboxylicacids having one carbon atom less than that of carbonyl compound. The methyl group is converted to haloform. This oxidation does not affect a carbon-carbon double bond, if present in the molecule.
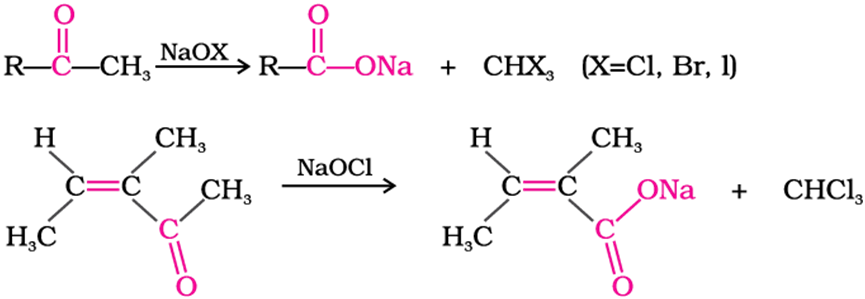
Reactions due to alpha-hydrogen
Aldol condensation of ketone
ketones having at least oneα-hydrogen undergo a reaction in the presence of dilute alkali as catalyst to formβ-hydroxy aldehydes (aldol) orβ-hydroxy ketones (ketol), respectively. This is known asAldol reaction.
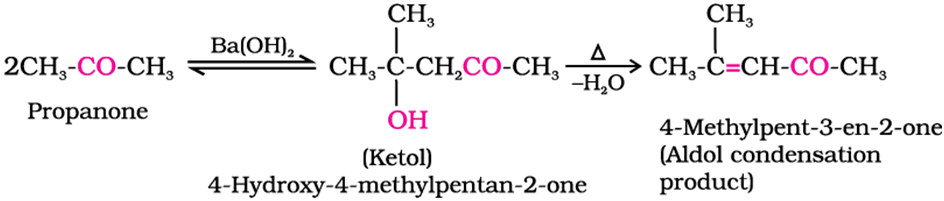
Cross aldol condensation of ketone
Ketones can also be used as one component in the cross-aldol reactions.

Cannizzaro reaction of ketone
· Cannizzaro reaction is not applicable to ketones
· Ketones do not have an alpha-hydrogen atom that is necessary for the deprotonation step to occur, and as a result, they cannot undergo the Cannizzaro reaction.
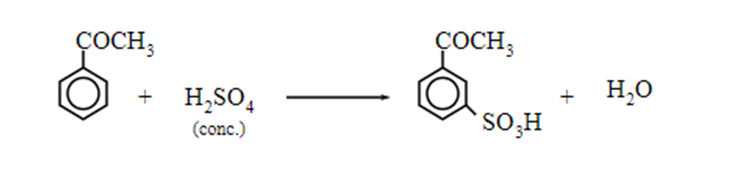
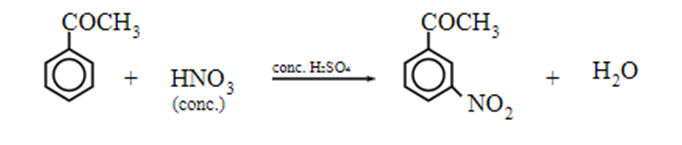
Electrophilic substitution reaction of ketone
Aromatic ketones undergo electrophilic substitution reactions such as nitration, sulphonation and halogenation. Since the ketonic group (-COR or -COAr) are electron-withdrawing, they are deactivating and m-directing.
Nitration
Sulphonation