Integrated Rate Equations
Zero Order Reactions
Zero-order reaction means that the rate of the reaction is proportional to zero power of the concentration of reactants. Consider the reaction,
Rate=
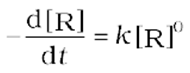
As any quantity raised to power zero is unity
Rate=
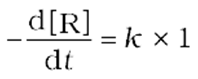
Integrating both sides
Att= 0, the concentration of the reactant R = [R]0, where [R]0 is the initial concentration of the reactant.
Substituting in equation (5)
[R]0= –k× 0 + I
[R]0= I
Substituting the value of I in the equation (5)
[R] = -kt+ [R]0 (6)
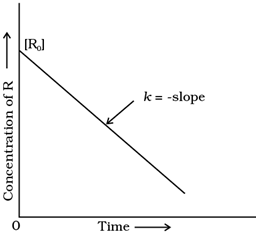
Variation in the concentration vs. time plot for a zero-order reaction
Comparing (6) with the equation of a straight line, y = mx + c, if we plot [R] against t, we get a straight line with slope = –k and intercept equal to [R]0.
Further simplifying equation (4.6), we get the rate constant,kas
![]() (7)
(7)
Zero order reactions are relatively uncommon but they occur under special conditions. Some enzyme catalysed reactions and reactions which occur on metal surfaces are a few examples of zero-order reactions. The decomposition of gaseous ammonia on a hot platinum surface is a zero-order reaction at high pressure.
![]()
Rate =k[NH3]0=k
In this reaction, platinum metal acts as a catalyst. At high pressure, the metal surface gets saturated with gas molecules. So, a further change in reaction conditions is unable to alter the amount of ammonia on the surface of the catalyst making rate of the reaction independent of its concentration. The thermal decomposition of HI on gold surface is another example of zero order reaction.
First Order Reactions
In this class of reactions, the rate of the reaction is proportional to the first power of the concentration of the reactant R. For example,
![]()
or
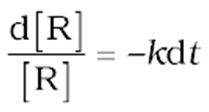
Integrating this equation, we get
ln [R] =–kt+ I (8)
Whent= 0, R = [R]0, where [R]0is the initial concentration of the reactant.
Therefore, equation (8) can be written as
ln [R]0= –k× 0 + I
ln [R]0= I
Substituting the value of I in equation (8)
Rearranging this equation
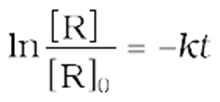
or
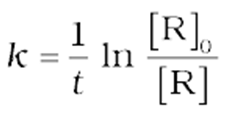 (10)
(10)
*ln[R]1= –kt1+ *ln[R]0 (11)
ln[R]2= –kt2+ ln[R]0 (12)
where [R]1and [R]2are the concentrations of the reactants at timet1andt2respectively.
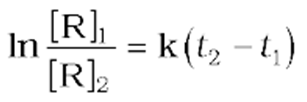 (13)
(13)
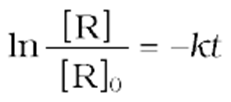
Taking antilog of both sides
Comparing equation (9) with y = mx + c, if we plot ln [R] against t we get a straight line with slope = –k and intercept equal to ln [R]0
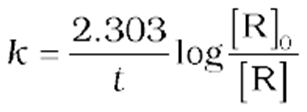 (15)
(15)
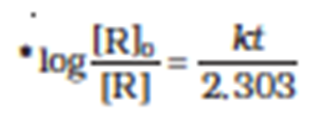
If we plot a graph between log [R]0/[R] vs t, the slope = k/2.303
C2H4(g) + H2(g)→C2H6(g)
Rate =k[C2H4]
All natural and artificial radioactive decay of unstable nuclei takes place by first-order kinetics.
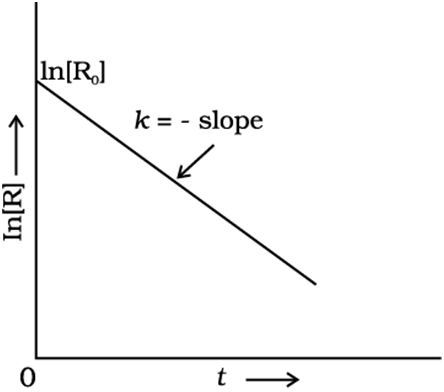
A plot between ln[R] and t for a first-order reaction
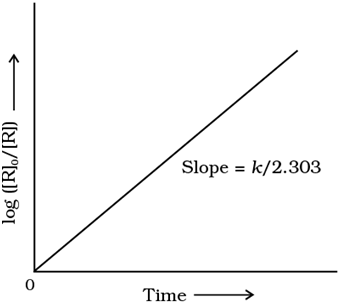
Plot of log [R]0/[R] vs time for a first order reaction
![]()
Rate =k[Ra]
Decomposition of N2O5and N2O are some more examples of first-order reactions.

where piis the initial pressure at timet= 0.
= 2pi–pt
k =
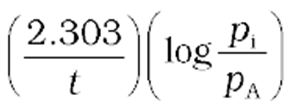 (16)
(16)
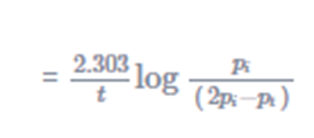
Half-Life of a Reaction
The half-life of a reaction is the time in which the concentration of a reactant is reduced to one-half of its initial concentration. It is represented as t1/2.
For a zero-order reaction, the rate constant is.
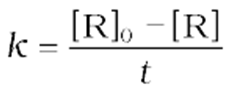
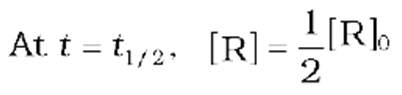
The rate constant at t1/2becomes
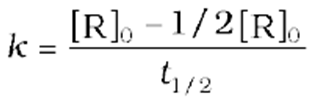
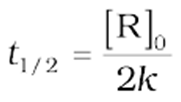
It is clear thatt1/2for a zero-order reaction is directly proportional to the initial concentration of the reactants and inversely proportional to the rate constant.
For the first-order reaction,
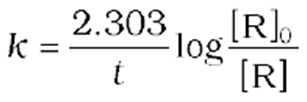
att1/2 ![]()
So, the above equation becomes
![]()
or
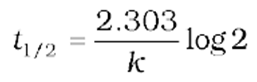
![]()
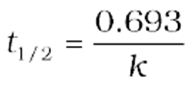
It can be seen that for a first-order reaction, the half-life period is constant, i.e., it is independent of the initial concentration of the reacting species. The half-life of a first-order equation is readily calculated from the rate constant and vice versa.
For zero order reactiont1/2∝[R]0. For first-order reaction, t1/2is independent of [R]0.
Pseudo-first-order reactions
Pseudo-first-order reactions are not truly first-order but show first-order kinetics under certain conditions. e.g., hydrolysis of ester, inversion of cane sugar
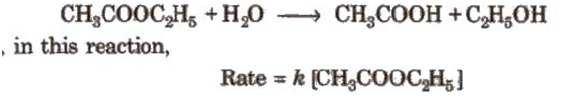
If water is present in excess amounts.