GROUP 13 ELEMENTS: THE BORON FAMILY
Boron is a typical non-metal, aluminium is a metal but shows many chemical similarities to boron, and gallium, indium and thallium are almost exclusively metallic in character.
Boron is a fairly rare element, mainly occurs as orthoboric acid, (H3BO3), borax, Na2B4O7·10H2O, and kernite, Na2B4O7·4H2O.
There are two isotopic forms of boron 10B (19%) and 11B (81%).
Aluminium is the most abundant metal and the third most abundant element in the earth’s crust (8.3% by mass) after oxygen (45.5%) and Si (27.7%).
Bauxite, Al2O3. 2H2O and cryolite, Na3AlF6are the important minerals of aluminium. In India it is found as mica in Madhya Pradesh, Karnataka, Orissa and Jammu. Gallium, indium and thallium are less abundant elements in nature.
Electronic Configuration of boron family
The elements of group 13 belong to p-block of the periodic table and these elements contain three electrons in the valence shell, therefore, their valence shell electronic configuration is ns2np1.
|
Element |
At. No. |
Electronic Configuration. |
Valence Shell Configuration. |
|
B |
5 |
[He] 2s2, 2p1 |
2s22p1 |
|
Al |
13 |
[Ne] 3s2, 3p1 |
3s23p1 |
|
Ga |
31 |
[Ar]3 3d10, 4s24p1 |
4s24p1 |
|
In |
49 |
[Kr] 4d10, 5s25p1 |
5s25p1 |
|
Tl |
81 |
[Xe] 4f14, 5d10, 6s2p1 |
6s26p1 |
Atomic Radii of boron family
On moving down the group, for each successive member one extra shell of electrons is added and, therefore, atomic radius is expected to increase. However, a deviation can be seen. Atomic radius of Ga is less than that of Al. This can be understood from the variation in the inner core of the electronic configuration. The presence of additional 10d-electrons offer only poor screening effect for the outer electrons from the increased nuclear charge in gallium. Consequently, the atomic radius of gallium (135 pm) is less than that of aluminium (143 pm).
Ionization Enthalpy of boron family
The first I.E. values of group 13 elements are lower than the corresponding values of the alkaline earth metals, due to the fact that removal of electron is easy in former case (p-electron) than latter (s-electron).
This results in the increase of nuclear charge. Consequently the valence electrons are more tightly held leading to high I.E. Similarly we can explain the irregularity in case of Tl on the basis of ineffective shielding of intervening electrons
On moving down the group IE, decreases from B to Al but the next element Ga has slightly higher IE, than Al, it again decreases in In and increases in the last element Tl as follows :
|
Element |
B |
Al |
Ga |
In |
Tl |
|
IE, (kJ mol–1) |
800 |
577 |
578 |
558 |
590 |
The irregularity observed in case of Gallium is due to the ineffective shielding of nuclear charge because of intervening d electrons, which cause the increase in nuclear charge leading to high I.E.
Electronegativity of boron family
Down the group, electronegativity first decreases from B to Al and then increases marginally. This is because of the discrepancies in atomic size of the elements.
Physical Properties of boron family
Boron is non-metallic in nature. It is extremely hard and black coloured solid. It exists in many allotropic forms. Due to very strong crystalline lattice, boron has unusually high melting point. Rest of the members are soft metals with low melting point and high electrical conductivity.
DENSITY
It increases regularly on moving down the group from B to Tl
MELTING AND BOILING POINTS
M.P. and b. p. of group 13 elements are much higher than those of group 2 elements
|
Element |
B |
Al |
Ga |
In |
Tl |
|
M.p. (K) |
2453 |
933 |
303 |
430 |
576 |
|
B.p. (K) |
3923 |
2740 |
2676 |
2353 |
1730 |
The m.p. decreases from B to Ga and then increases, due to structural changes in the elements
Boron has a very high m. p. because of its three dimensional (B12-icosahedral) structure in which B atoms are held together by strong covalent bonds.
Low m. p. of Ga is due to the fact that it consists of only Ga2 molecules, and Ga remains liquid upto 2273K therefore it is used in high temperature thermometry.
Chemical Properties of boron family
Oxidation state and trends in chemical reactivity
Inert pair effect
It is the reluctance of the s-electrons of the valence shell to take part in bonding and occurs due to ineffective shielding of the ns2 electrons by the intervening d and f electrons.
It increases down a group and thus the lower elements of group show lower oxidation states.
OXIDATION STATES
· B and Al show an oxidation state of +3 only while Ga, In and Tl show oxidation states of both +1 and +3.
· As we move down in the group 13, due to inert pair effect, the tendency to achieve +3 oxidation state goes on decreasing and the tendency to acquire +1 oxidation state goes on increasing.
· Stability of +1 oxidation state follows the order Ga < In < Tl
· Tl+ compounds are more stable than Tl3+ compounds.
Reactivity group 13 elements towards air
Crystalline boron is unreactive whereas amorphous boron is reactive. It reacts with air at 700°C as follows
4B + 3O2→ 2B2O3
2B + N2→ 2BN
AI is stable in air due to the formation of protective oxide film.
4Al + 3O2→ 2Al2O3
Thallium is more reactive than Ga and In due to the formation of a unipositive ion, TI+.
4Tl + O2→ 2Tl20
Reactivity group 13 elements towards acids and alkalies
Reaction with alkaliesBoron dissolves in alkalies and gives sodium borates.

Aluminium also reacts with alkali and liberates hydrogen.
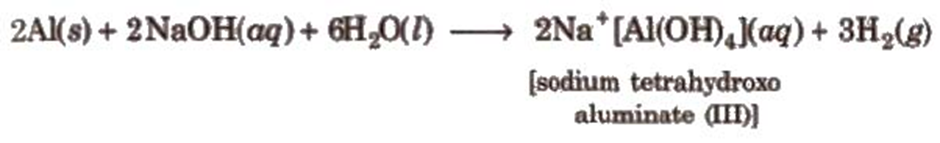
Reactivity group 13 elements towards halogens
PREPARATION OF HALIDES
All the elements of boron family (except thallium which forms thallous monohalides ) form trihalides of type MX3 where X= F, Cl, Br and I.
B2O3 + 3C + 3Cl2 ![]() 2BCl3 + 3CO
2BCl3 + 3CO
Al2O3 + 3C + 3Cl2 ![]() 2AlCl3 + 3CO
2AlCl3 + 3CO
All the boron trihalides, BX3 and aluminium trihalides AlX3 (except AlF3 which is ionic) are covalent compounds whereas former exist as only monomers and latter as dimers, because boron atom is too small to coordinate with four large halide ions and in case of much smaller F– ion , the energy released during the formation of the bridge structure is not sufficient for the cleavage of the typical p![]() -p
-p![]() bond in BF3.
bond in BF3.
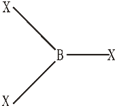 BX3
BX3
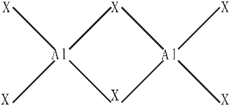 Al2X6
Al2X6
BF3 is a colourless gas, BCl3 and BBr3 are colourless fuming liquids whereas BI3 is a white fusible solid at room temperature.
The covalent character of trihalides decreases on moving from Ga to Tl.
Hybridisation of Boron in BCl3 is sp2
NATURE OF TRIHALIES
Trihalides of group 13 elements behave as lewis acids due to having a strong tendency to accept a pair of electrons.
The relative strength of lewis acids of boron trihalides increases in the order :
BF3 < BCl3 < BBr3 < BI3.
The halides of group 13 elements behave as lewis acids and the acidic character decreases as follows:
BX3 > AlX3 > GaX3 > InX3 (where X=Cl, Br or I)
BF3 and anhydrous AlCl3 are used as a catalyst in Friedel Crafts reactions.
TlCl3 decomposes to TlCl and Cl2 above 40ºC and hence acts as an oxidising agent, whereas TlBr3 converts into Tl[TlBr4] at room temperature.
TlCl3 ![]() TlCl + Cl2 ;2TlBr3
TlCl + Cl2 ;2TlBr3 Tl[TlBr4] +Br2
Tl[TlBr4] +Br2
While TlI3 is an ionic compound containing Tl(I) and ![]() ions.
ions.
Anomalous properties of boron
Boron shows anomalous behaviour with the other members of the group, due to the following reasons:
(i) Smallest size in the group.
(ii) High ionisation energy.
(iii) Highest electronegativity in the group.
(iv) Absence of vacant d-orbital.
A few points of difference are
1. It is a non-metal while other members of the group are metallic.
2. It shows allotropy while other members do not.
3. It has the highest melting point and boiling point in group 13.
4. It forms only covalent compounds while other members form both ionic and covalent compounds.
5. The halides of boron exist as monomers while AlCI:! exists as a dimer.
6, The oxides and hydroxides of boron are weakly acidic while those of aluminium arc amphoteric and those of other elements are basic.
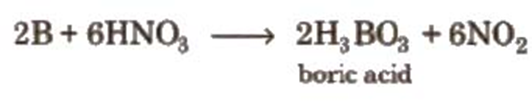
7. It can be oxidised by concentrated HNO3while aluminium becomes passive due to the formation of oxide layer on the surface.