ELECTRONIC CONFIGURATION OF ELEMENTS
Periodic table Groups
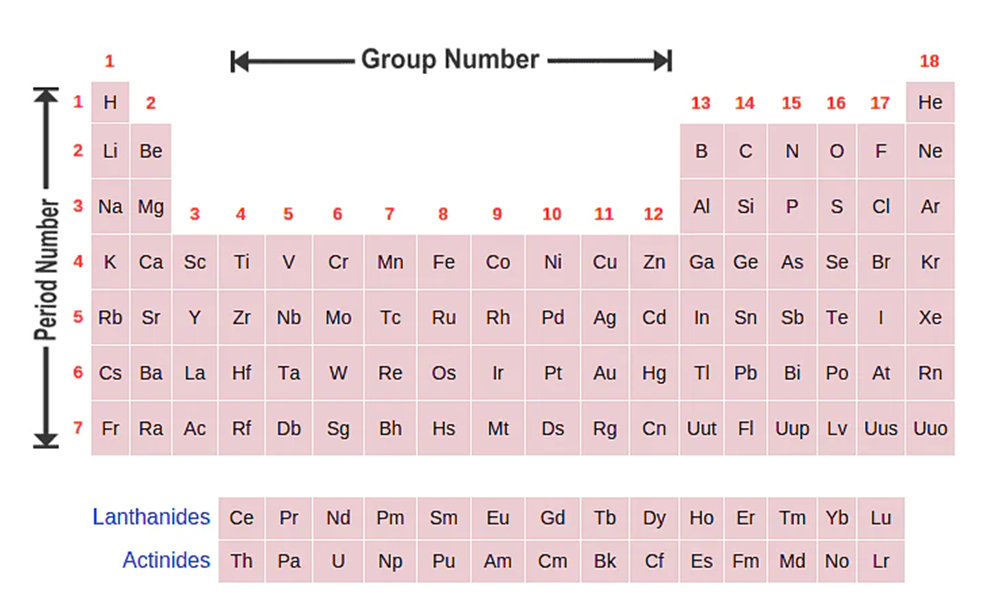
- There are 18 vertical columns in the periodic table. Each column is called a group.
- All elements in a group have similar chemical and physical properties because they have the same number of outer electrons.
- If the element is in s block, then the group number is equal to the number ofvalence electrons.
- If the element is in the p block, then the number of the group can be determined by the formula:- (number of valence electrons + 10).
Periodic table Period
- Periods are the horizontal rows of elements in a periodic table.
- There are seven periods in the periodic table.
- The period number is related to the number of electron-occupied shells in the element.
- For example, If an element has electronic configuration 2,5. This shows that the number of electron-occupied shells in the element is 2. Hence this element belongs to 2ndperiod.
Number of Elements in a Period
|
Period Number |
Number of Elements |
|
1 |
2 |
|
2 |
8 |
|
3 |
8 |
|
4 |
18 |
|
5 |
18 |
|
6 |
32 |
|
7 |
32(incomplete) |
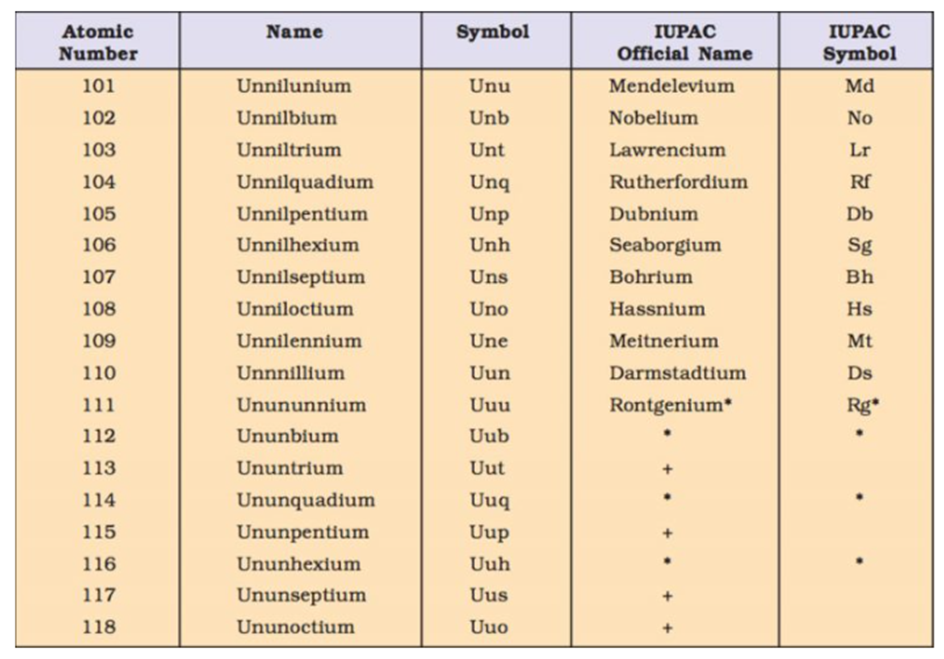
NOMENCLATURE OF ELEMENTS WITH ATOMIC NUMBER >100
|
Digit |
Name |
Abbreviation |
|
0 |
nil |
n |
|
1 |
un |
u |
|
2 |
bi |
b |
|
3 |
tri |
t |
|
4 |
quad |
q |
|
5 |
pent |
p |
|
6 |
hex |
h |
|
7 |
sept |
s |
|
8 |
oct |
o |
|
9 |
enn |
e |
EXAMPLE-
ELECTRONIC CONFIGURATIONS OF ELEMENTS AND PERIODIC TABLE
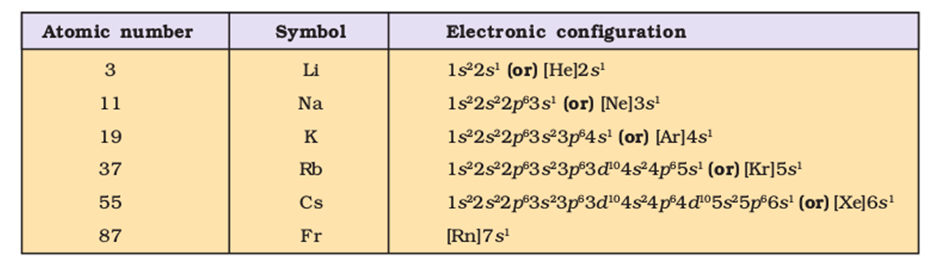
Electronic Configurations in Periods
Each period in the periodic table indicates the value of n for the outermost or the valence shell. The total number of elements in each period is twice the number of orbitals available in the energy level that is being filled.
(1) Thefirst period corresponds to the filling of electrons in the first energy shell i.e. (k shell),n=1. Since this energy shell has only 1 orbital i.e. 1s which can accommodate only 2 electrons, therefore, first period has only 2 elements.
(2) Thesecond period corresponds to the filling of electrons in the second energy shell (L shell) i.e. n=2. This shell has 4 orbitals( one 2s and three 2p) which can accommodate 8 electrons, therefore second period contains 8 electrons. It starts with Lithium (Z=3) and ends at neon (Z= 10).
(3) Thethird period corresponds to the filling of electron in the third shell, i.e. n=3. This shell has 9 orbitals ( one 3s, three 3p and five 3d) .3d orbital have even higher energy than 4s orbital. Therefore 3d orbitals are filled only after filling of 4s orbital. The third period involves the filling of only 4 orbitals( one 3s and three 3p) and thus contains 8 elements. It starts with sodium(Z=11) and ends at argon (Z= 18).
(4) TheFourth periodcorresponds to the filling of electrons in the fourth energy level, n=4. It starts with potassium( Z=19) and ends at calcium (Z= 20).
After the filling of 4s orbitals, the filling of five 3d orbitals begins since the energy of 3d orbital is lower than those of 4p orbitals but higher than that of 4s orbital. The filling of 4d and 4f orbital does not occur in this period since their energies are higher than that of even 5s orbital. The filling of the 3d orbital starts from scandium( Z= 21) and ends at Zinc( Z= 30).These 10 elements constitute the3d transition series.
The filling of 4p orbital begins at gallium( Z=31)and ends at krypton( Z=36) which has the outer electronic configuration as 4s23d104p6.In the 4th period, the filling of only 9 orbitals( one 4s, five 3d and three 4p ) occurs which can accommodate at the maximum 18 electrons. Therefore 4th period contain 18 electrons from potassium to Krypton.
(5) Thefifth periodalso contains 18 elements since only 9 orbitals ( one 5s, five 4d and three 5p ) are available for filling with electrons. It begins with rubidium(Z= 37) in which one electron enters 5s orbital. After the filling of 5s orbital, the filling of 4d orbital starts at yittrium (Z=39) and ends at cadmium (Z= 48).These ten elements constitute4d transition series. Filling of 5p orbitals starts at indium (Z= 49) and ends at xenon ( Z=54).
(6) Thesixth period corresponds to the filling of 6th energy level i.e. n= 6.Only 16 orbitals( one 5s, five 4d and three 5p) are available for filling with electrons, therefore 6th period contains 32 elements. It begins with caesium(Z=55) in which one electron enters the 6s orbital and ends up with radon(Z=86) in which the filling of 6p orbital is complete. After the filling of 6s Orbital, the next electron enters the 5d orbital and therefore the filling of seven 4f orbitals begins with Cerium(Z=58) and ends up with lutetium(Z=71).These 14 elements constitute the first inner transition series called lanthanides or lanthanoids.
Filling of 5d orbitals which started at lanthanum continues from hafnium( Z=72) till it is filled at mercury(Z=80). These 10 elements constitute the 5d- transition series. After the filling of 5d orbitals, the filling of 6p orbitals starts at thallium(Z=81) and ends at the radon (Z=86).
(7) The seventh period corresponds to filling of 7th energy shells i.e. n=7. It also contain 32 elements corresponds to the filling of 16 orbitals(one 7s, seven 5f, five 6d and three 7p ).
After the filling of 7s orbital, the next two electrons enters the 6d orbitals and therefore the filling of seven 5f orbitals begin with proactinium(Z=91) and ends up with lawrencium(Z=103).
Thorium does not have any electron in the 5f orbital, yet it is considered to be a f-block element since its properties resemble more the f block element than the d block elements. These 14 elements from thorium(Z=90) to lawrencium(Z=103) constitute the second (or 5f) inner transition serieswhich is called asactinides are actinoids.
Filling of 5d orbitals which started at actinum(Z=89) continues till it is completed at these Uub(Z=112). These 10 elements constitute the 6d transition series. The filling of 6d, orbital the filling of 7p orbitals begins at Uut (Z= 118) and ends at Uut (Z=118) which belongs to the noble gas family.
The first three periods containing 2,8,8 elements and are known asshort periods while the next three periods containing 18 ,18, and 32 elements are called Long periods
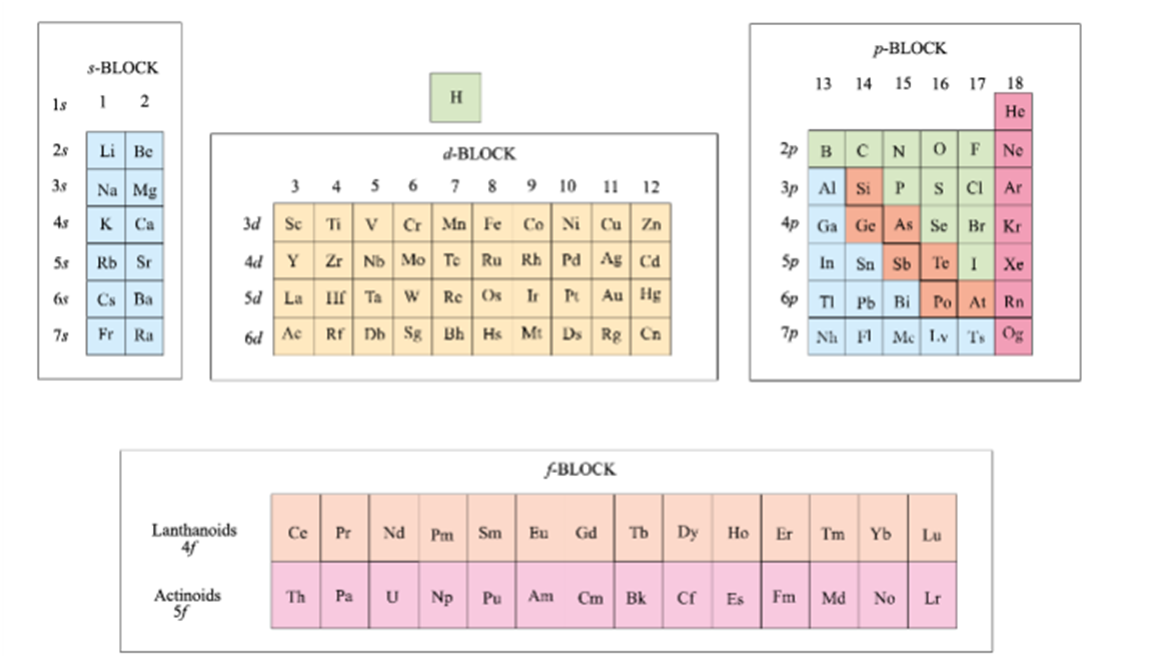
Groupwise Electronic Configurations
In group 1, the electronic configuration is 1s2. This means that there are two electrons in the outermost energy level. In group 2, the electronic configuration is 2s2. This means that there are two electrons in the outermost energy level and two electrons in the second outermost energy level. In group 3, the electronic configuration is 3s2. This means that there are two electrons in the outermost energy level, two electrons in the second-outermost energy level, and two electrons in the third-outermost energy level. In group 4, the electronic configuration is 4s2. This means that there are two electrons in the outermost energy level, two electrons in the second-outermost energy level, two electrons in the third-outermost energy level, and two electrons in the fourth-outermost energy level. In group 5, the electronic configuration is 5s2. This means that there are two electrons in the outermost energy level, and two electrons in the second-outermost energy level, and two electrons in the third-outermost energy level, and two electrons in the fourth-outermost energy level, and two electrons in the fifth-outermost energy level. In group 6, the electronic configuration is 6s2. This means that there are two electrons in the outermost energy level, and two electrons in the second-outermost energy level, and two electrons in the third-outermost energy
ELECTRONIC CONFIGURATIONS AND TYPES OF ELEMENTS
|
Group 1 |
Alkali metals ( s – s-block element) |
· Outermost electronic configuration – ns1 · The elements in the first group, lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr) are called alkali metals. · They were given the name because they all react with water to form alkalis. · The alkali metals are all shiny, soft, highly reactive solids at standard temperature and pressure. · They lose the outermost electron(s) readily to form 1+ ion. · Number of valence electrons = 1 |
|
Group 2 |
Alkaline earth metals (s-block element) |
· Outermost electronic configuration – ns2 · The elements in the second group, beryllium(Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra) are called alkaline earth metals. · They were given the name because their oxides are alkaline in nature. · They are all shiny, silvery-white, somewhat reactive hard solids at standard temperature and pressure. · They lose the outermost electron(s) readily to form +2 ion. · Number of valence electrons = 2 |
The p-Block Elements
|
Group 13 |
Boron family or icosagens (p-block elements) |
· Outer electronic configuration – ns2np1 · Group 13 elements areboron (B), aluminum (Al), gallium (Ga), indium (In), thallium (Tl),andelement 113(briefly namedununtrium [Uut]). · Aluminium is the third most abundant element in the earth’s crust. · Ununtrium is been never found in natureand has been named asynthetic element. · Number of valence electrons – 3 · Valency – 3 |
|
Group 14 |
Carbon family or tetragens(p – block elements) |
· Outermost electronic configuration – ns2np2 · Group 14 elements are Carbon, silicon, germanium, tin, and lead. · Silicon is the second most common element found in the earth’s crust (after oxygen). · Number of valence electrons- 4 · Valency -4 |
|
Group 15 |
pnicogens (p-block element) |
· The elements in the 15th group are Nitrogen(N), P (phosphorous), Arsenic (As), Antimony(Sb), Bismuith (Bi) · Number of valence electrons -5 · Valency -3 |
|
Group 16 |
chalcogens (p-block element) |
· Elements are oxygen(O), Sulfur(S), Selenium(Se), tellurium(Te), polonium(Po) · number of valence electrons -6 · Valency -2 |
|
Group 17 |
halogens (p-block element) |
· The elements in the seventeenth group (F, Cl, Br, I and As) are exist as diatomic molecules. · The halogens exist at room temperature in all three states of matter: Solid – Iodine, Astatine. Liquid – Bromine. Gas – Fluorine, Chlorine. · Number of valence electrons = 7 · Valency -1 |
|
group 18 |
noble gas (p-block element) |
· The elements in the eighteenth group, helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and the radioactive radon (Rn) are called noble gases. · They are all odourless, colourless and monatomic gases with very low chemical reactivity. · Since their valence shell is considered to be “full”, they have little tendency to participate in chemical reactions. · Number of valence electrons = 8 · Valency -0 |
The d-Block Elements
|
Group 3 to 12 |
Transition elements(d – block elements) |
|
The f-Block Elements
|
Inner transition elements |
f – block elements |
· The two rows of elements at the bottom of the Periodic Table, called the Lanthanoids, Ce(Z = 58) – Lu(Z = 71) and Actinoids, Th(Z = 90) – Lr (Z = 103). · Outer electronic configuration – (n-2)f1-14(n-1)d0-1ns2 · They are all metals. · They are generally hard. · F-block elements display variable oxidation states. · Most f-block elements have high melting points. · Many of the f-block elements (the actinides) are radioactive. · The elements after uranium are called Transuranium Elements. |
Metals, Non-metals and Metalloids
The elements are classified intos-,p-,d-, andf-blocks. The elements can be divided intoMetalsandNon-Metals. Metals comprise more than 78% of all known elements and appear on the left side of thePeriodic Table. Metals are usually solids at room temperature [mercury is an exception; gallium and caesium also have very low melting points (303K and 302K, respectively)]. Metals usually have high melting and boiling points. They are good conductors of heat and electricity. They are malleable (can be flattened into thin sheets by hammering) and ductile (can be drawn into wires). In contrast, non-metals are located at the top right hand side of thePeriodic Table. In fact, in a horizontal row, the property of elements change from metallic on the left to non-metallic on the right. Non-metals are usually solids or gases at room temperature with low melting and boiling points (boron and carbon are exceptions). They are poor conductors of heat and electricity. Most non-metallic solids are brittle and are neither malleable nor ductile. The elements become more metallic as we go down a group; the non-metallic character increases as one goes from left to right across thePeriodic Table. The change from metallic to non-metallic character is not abrupt. The elements (e.g., silicon, germanium, arsenic, antimony and tellurium) bordering this line and running diagonally across the Periodic Tableshow properties that are characteristic of both metals and non-metals. These elements are calledSemi-metalsorMetalloids.