Electrochemical Cells
|
Electrochemical cell 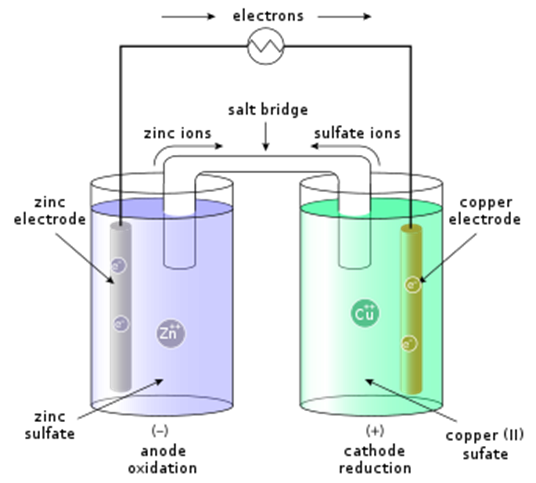 |
Electrochemical cell is also known asgalvanic cell. Electrochemical cellconverts the chemical energy of a spontaneous redox reaction into electrical energy. Electrons flows from anode to cathode. Anode – oxidation takes place – negatively charged. Cathode – reduction takes place – positively charged electrolyte. |