BATTERIES
Primary cell
- In these cells, reactions occur only once and after use they become dead. Therefore, they are not chargeable.
- Some common examples are dry cell, mercury cell.
- Dry cells – They are used in transistors and clocks. They are also known as Leclanche cell.
- Mercury cells – They are used in low current devices likehearing aids, watches, etc.
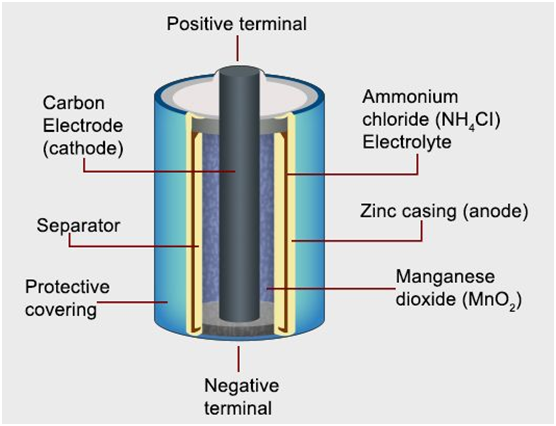
Dry cell
- It is a type ofprimary battery.
- In the primary batteries, the reactionoccurs only once, and after use over a period of time battery becomes dead and cannot be reused again.
- The cell consists of azinc container that also acts as an anode and the cathode is a carbon (graphite) rod surrounded by powdered manganese dioxide and carbon.
- The space between the electrodes is filled with a moist paste ofammonium chloride (NH4Cl) and zinc chloride (ZnCl2).
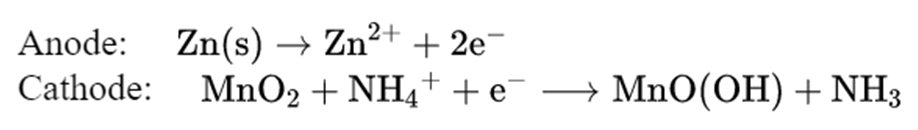
Mercury cell :
- It consists of zinc – mercury amalgam as anode and a paste of HgO and carbon as the cathode.
- The electrolyte is a paste ofKOH and ZnO.
- The overall reaction is represented by Zn(Hg) + HgO(s)→ ZnO(s) + Hg(l)
- The cell potential is approximately 1.35 Vand remains constant during its life as the overall reaction does not involve any ion in solution whose concentration can change during its life a time.

Mercury cell
Mercury cell, suitable for low current devices like hearing aids, watches, etc. consists of zinc – mercury amalgam as anode and a paste of HgO and carbon as the cathode. The electrolyte is a paste of KOH and ZnO. The electrode reactions for the cell are given below:
Anode:Zn(Hg) + 2OH–→ZnO(s) + H2O + 2e–
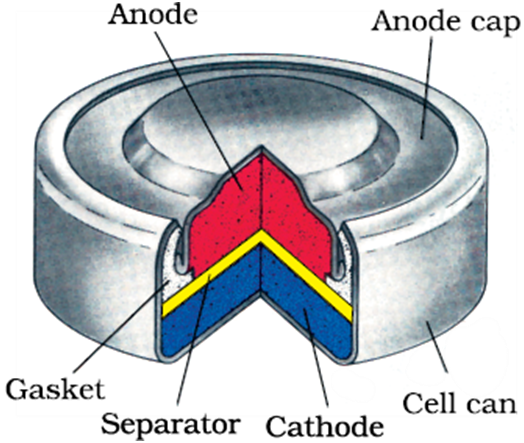
The overall reaction is represented by
Zn(Hg) + HgO(s)→ZnO(s) + Hg(l)
The cell potential is approximately 1.35 V and remains constant during its life as the overall reaction does not involve any ion in solution whose concentration can change during its life time.