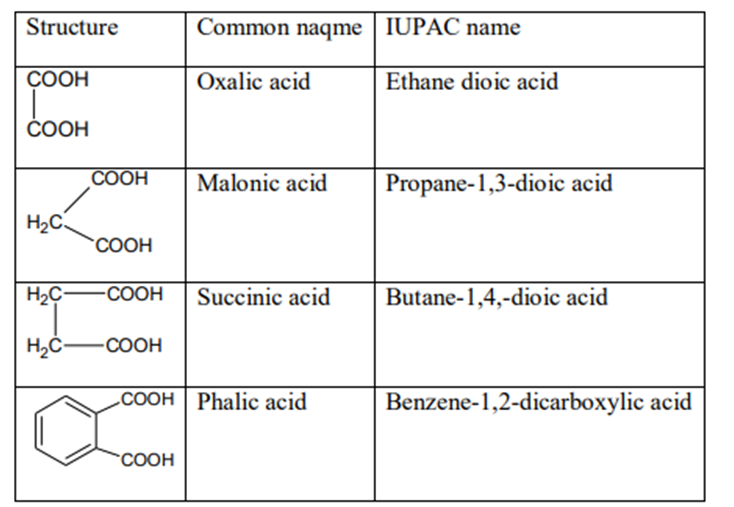
DICARBOXYLIC ACID
Carboxylic acid containing two –COOH grs. is called dicarboxylic acid. In IUPAC they are named by placing suffiw ‘dioic acid’ to the name of parent alkane.
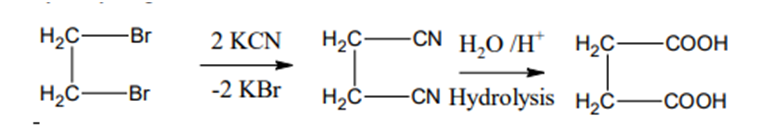
Preparation
From ethylene dibromide
Ethylene dibromide on treatment with KCN followed by hydrolysis gives succinic acid
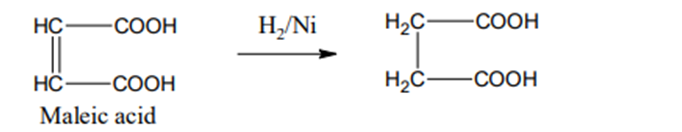
From Maleic acid
Maleic acid on reduction with H2 in presence of Ni gives, succinic acid.
Chemical properties
1) Action of heat
Succinic acid on heating above its melting point undergo dehydration to give corresponding anhydride.
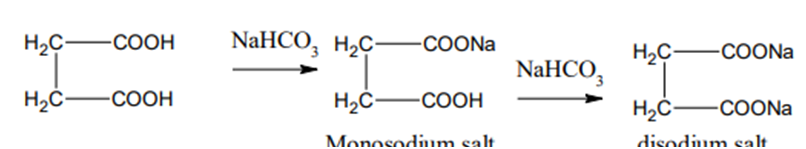
2) Action of NaHCO3
Succinic acid on treatment with sodium bicarbonate forms its sodium salt.
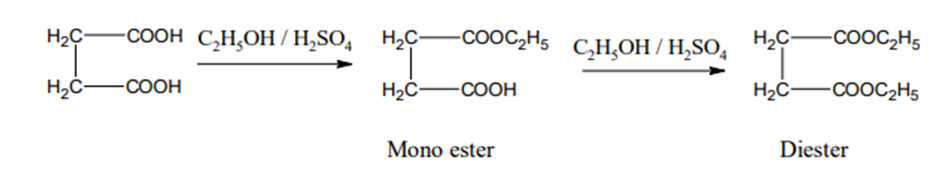
3) Action of Ethanol
In presence of acid succinic acid reacts with ethanol to give mono as well as diester.
Uses
It is used as, i) In manufacture of dyes and perfumes ii) In medicine iii) As laboratory reagent in volumetric analysis
Acidic strength of dicarboxylic acid
The dicarboxylic acids are stronger than monocarboxylic acid becasue it has a electron withdrawing (−COOH) group.
The increase in theCH2group in the compound will decrease the acidic strength of the compound becauseCH2is an +I effect group so it will increase the electron density on conjugate base.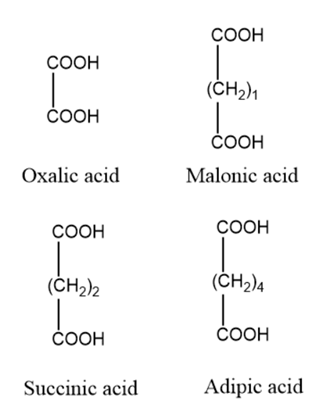
Here, oxalic acid doesn't have +I group so it more acidic.
Then, malonic acid has oneCH2and succinic acid has 2CH2and adipic acid has 4CH2.
Therfore the strength of acid follows the order,
Adipic acid