CYANIDES AND ISOCYANIDES
Both alkyl cyanides (RCN) and alkyl isocyanides (RNC) are organicderivatives of hydrocyanic acid HCN. Alkali cyanides are ionic ![]() and cyanide ion is ambident in nature (can form covalent bond either from carbon or nitrogen).AgC = Nis covalent, hence lone pair on nitrogen is mainly available for covalent bond formation, resulting in predominant formation of isocyanides.
and cyanide ion is ambident in nature (can form covalent bond either from carbon or nitrogen).AgC = Nis covalent, hence lone pair on nitrogen is mainly available for covalent bond formation, resulting in predominant formation of isocyanides.
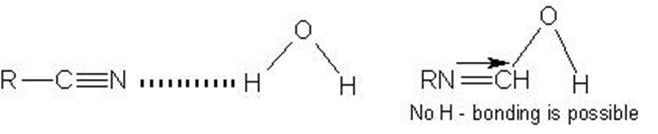
Illustration .How would you account for the fact that alkyl cyanides are soluble in water but alkyl isocyanides are insoluble in water?
Solution:Alkyl cyanides possess the tendency to form H – bonding with water which is absent with isocyanides
Methods of preparation of Cyanides
1.Dehydration of Amides:
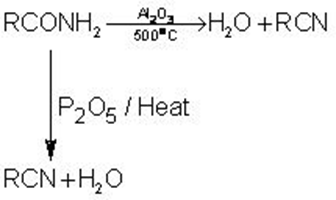
High molecular weight acid amides are dehydrated to the corresponding cyanide by heat alone.
CH3(CH3)6OCNH2  CH3(CH2)6CN
CH3(CH2)6CN
2. From RX:
RX + KCN ——> RCN + KX
This method is satisfactory only if R is1oor2ogroup. If it is3ogroup, then it is converted into alkene.
CH3CH2Cl + KCN → CH3CH2CN + KCl
3. By Grignard’s reagent and Cyanogen chloride reaction:
RMgCl + CICIN → RCN + MgCl2
This is best method for preparing3oalkyl cyanides.
(CH3)3CMgCl + CICN → (CH3)3CCN + MgCl2
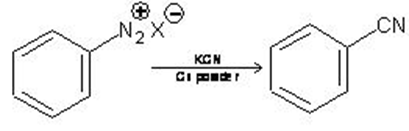
4. From Diazonium salt
Methods of Preparation of Isocyanides
1.By heating an alkyl iodide with AgCN in aqueous ethanolic solution
Rl + AgCN → RNC + Agl
C2H5l + AgCN → C2H5NC + Agl
Ethylisocyanide
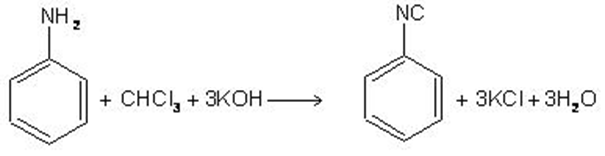
2.By carbylamine reaction
Heating a mixture of 1oamine and chloroform with ethanolic potassium hydroxide
RNH2+ CHCl2+ 4KOH ——> RNC + 3KCl + 3H2O
Mechanism proceeds via intermediate formation of dichloromethylene or, dichloro carbeneproduced from chloroform in alkaline solution. (Viaa-elimination)
CHCl3+ KOH ———>KCl + H2O + : CCl2
![]()
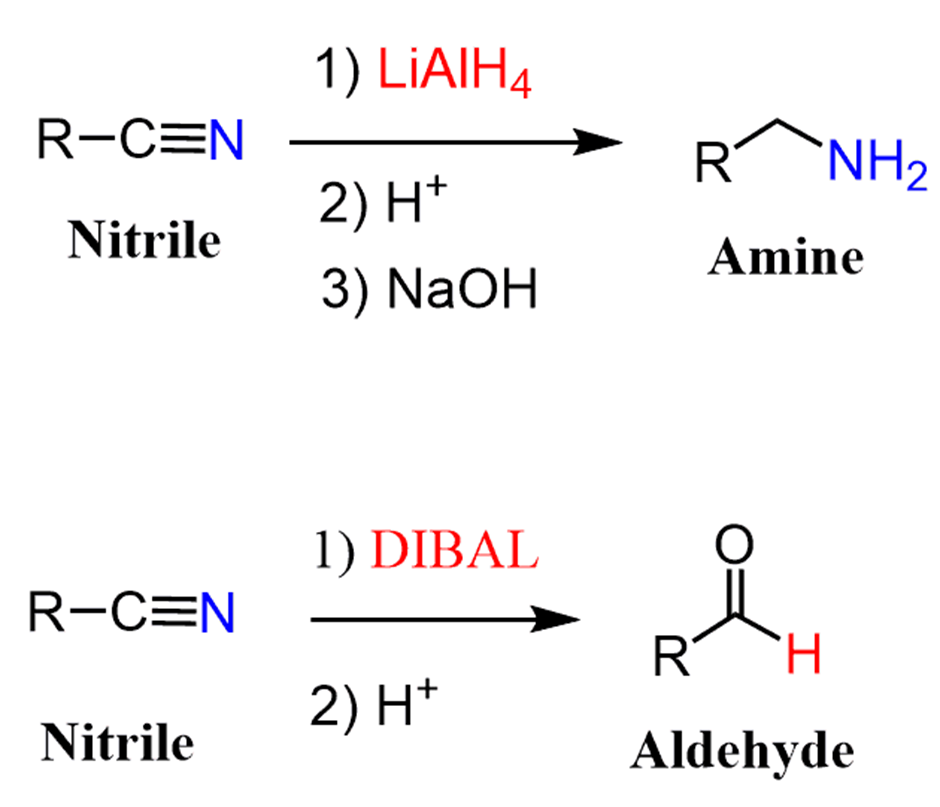
Reduction of nitriles
The reduction of nitriles using hydrogen and a metal catalyst.The carbon-nitrogen triple bond in a nitrile can also be reduced by reaction with hydrogen gas in the presence of a variety of metal catalysts. Commonly used catalysts are palladium, platinum or nickel.
Physical properties of cyanide and isocyanide
The lower alkyl cyanides and isocyanides are colourless liquids. Cyanides are highly polar compounds.Cyanides and isocyanides posses high boiling points.
The lower alkyl cyanides and isocyanides are colourless liquids while the higher members are crystalline solids.
Alkyl cyanides usally posses fairly pleasent odour,resembling that of bitter almonds
Isocyanides posses very unpleasent odour
- Cyanides are highly polar compounds and possess dipolemoments of the order of 4D.
- Isocyanides are also polar but they possess comparatively lesser dipolemoments.
- The dipole moments of isocyanides are of the order of 3D
Both cyanides and isocyanides possess high boiling points
· Their boiling points are higher than those of alkyl halides of comparable molecular masses
· Ex:355KCH3CN,332KCH3NC,249.5KCH3Cl
The lower alkyl cyanides are fairly soluble in water due to formation of hydrogen bonds
The solubility of alkyl cyanides in water decreases with increase in size of the alkyl group
Chemical properties of cyanide and isocyanide
1. Hydrolysis:
RNC + 2H2O 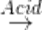 RNH2+ HCO2H
RNH2+ HCO2H
CH3NC + 2H2O 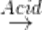 CH3NH2+ HCO2H
CH3NH2+ HCO2H
RNC are not hydrolysed by alkalis.
2. Reduction:
RNC R NHCH3
R NHCH3
2oamine
CH3NC  CH3NHCH3
CH3NHCH3
Methyl isocyanide Dimethyl amine
3. When alkyl isocyanides are heated for a long time, they arrange to form cyanide
RNC → R CN
CH3CH2NC → CH3CH2CN
4. With non metals:
(i) RNC + X2———> RNCX2
CH3NC + Cl2——> CH3NCCl2
(ii) RNC + S ———> RNCS
Alkyl isothiocyanates
CH3NC + S ———> CH3NCS
5. Oxidation with HgO:
RNC + HgO → RNCO + Hg
Akyl isocyanates
CH3NC + HgO → CH3NCO + Hg