Non stoichiometric defects
1. Metal excess defect
a) Anionic vacancies
Anion is missing from its lattice site and leaving hole which is trapped by an electron. the anion trap by electron is calledf centre
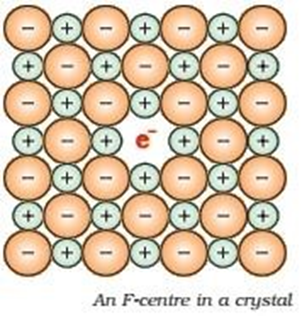
Why NaCl become yellow in presence of sodium vapours or why lithium chloride become pink in presence of lithium vapours or why potassium chloride become violet in presence of potassium vapours
Due To presence of f centre in this crystals. On absorption of visible light the electron can be excited and give colour
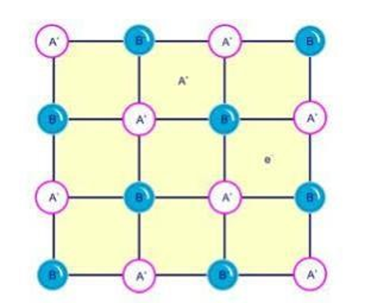
2.Due to presence of extra cation
•An extra cation occupy the interstitial site and another interstitial site is occupied by an electron
Why ZnO become yellow on heating
Zinc oxide is white in colour at room temperature. On heating it loses Oxygen and turn yellow. The excess Zn+2move to interstitial site and the electron to neighbouring interstitial site . on absorption of visible light electron excite and give colour
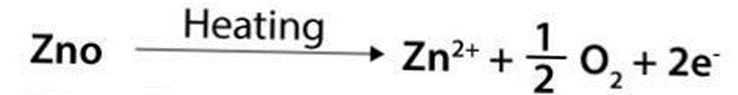
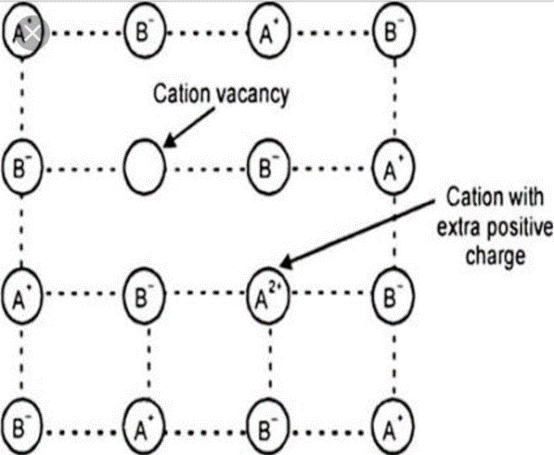
Metal deficiency defectit arise due to mising of cation and presence of cation having higher charge in the adjacent side
For Example : FeO, FeS etc