Crystal defects
Point defects – it arises due to irregularities of atom around a point
Line defect – it arise due to irregular writers of atoms in entire row of crystals
Types of point defects
- Stoichiometric defect
- Non Stoichiometric defect
- Impurity defect
Stoichiometric defect
The number of cations and anions are exactly same in ratio as indicated by ideal chemical composition
Stoichiometric defect in non ionic compound
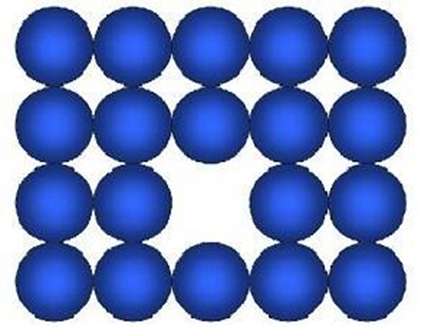
a) Vacancy defect
Some of the atoms leave their lattice site and create a vacancy in a crystal is called vacancy defect
The density decreases because mass decreases but volume remains same This effect arises when substance is heated
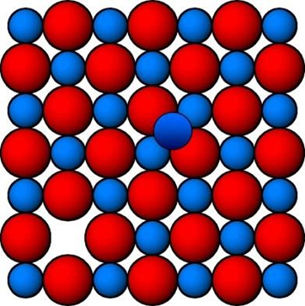
b) Interstitial defect
When some of the constituent particle occupy interstitial site is called interstitial defect
Density increases because mass increases and volume remains same
Stoichiometric defect in ionic compound
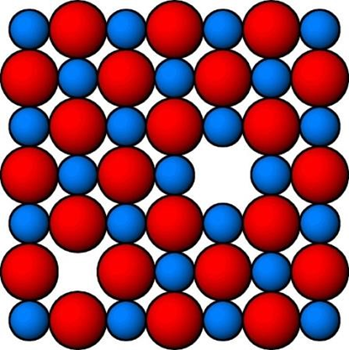
Difference between Frenkel and Schottky defect
Schottky defect |
Frenkel defect |
|
|
|
|
Same number of anion and cation are missing from their lattice site |
An ion (mostly cation due to small size )missing from their lattice site and occupied interstitial site in a crystal |
|
Electrical neutrality is maintained |
Electrical neutrality is maintained |
|
Density decrease because mass decrease due to missing of cation and anion from their |
Density remains same because no ions is missing |
|
lattice site and volume remains same |
|
|
Compounds exhibit schottky defect must have less difference in size of cation and anion |
Large difference in size of cation and anion |
|
Example NaCl , KCl , AgBr |
AgI , AgBr , AgCl , ZnS |
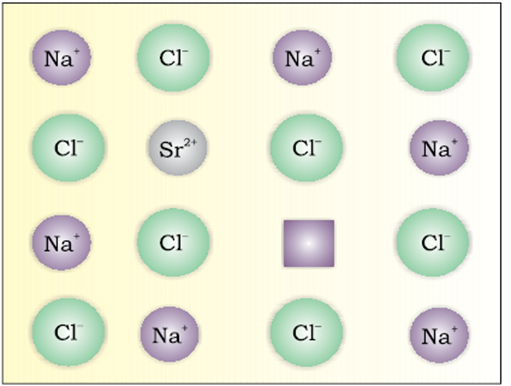
Impurity defect
If molten NaCl containing a little amount of SrCl2is crystallised, some of the sites of Na+ions are occupied by Sr2+. Each Sr2+replaces two Na+ions. It occupies the site of one ion and the other site remains vacant. The cationic vacancies thus produced are equal in number to that of Sr2+ions. Another similar example is the solid solution of CdCl2and AgCl.