Aluminium compounds
ANHYDROUS ALUMINIUM CHLORIDE, AlCl3 (or Al2Cl6)
PREPARATION
It can not be prepared by heating AlCl3. 6H2O because of its hydrolysing tendency by its own water as below.
2AlCl3. 6H2O 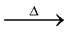 2Al(OH)3 + 6HCl
2Al(OH)3 + 6HCl
2Al(OH)3 ![]() Al2O3 + 3H2O
Al2O3 + 3H2O
However, it can be prepared by following methods:
By passing dry chlorine or HCl gas over heated Al.
2Al + 3Cl2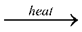 2AlCl3
2AlCl3
2Al + 6HCl 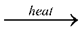 2AlCl3 + 3H2
2AlCl3 + 3H2
By heating a mixture of alumina and carbon in a current of dry chlorine.
Al2O3 + 3C + 3Cl2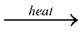 2AlCl3 + 3CO
2AlCl3 + 3CO
PROPERTIES
It fumes in moist air due to hydrolysis
AlCl3 + 3H2O  Al(OH)3 + 3HCl
Al(OH)3 + 3HCl
The resulting solution is acidic due to the formation of HCl.
It behaves as lewis acid.
It is a covalent solid and dissolves in organic solvents like C6H6 etc.
STRUCTURE
It exists as dimer Al2Cl6 in which each Al atom is tetrahedrally surrounded by four Cl atoms as below.
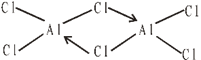
USES
· As a catalyst in Friedel - Craft reactions
· As a mordant in dyeing
ALUMINIUM OXIDE OR ALUMINA, Al2O3
It is the most stable compound of aluminium and occurs in nature as colourless corundum and several coloured oxides, (when present in combination with different metal oxides) like ruby (red), topaz (yellow), sapphire (blue), amethyst (violet) and emerald (green), which are used as precious stones (gems).
THERMITE
A mixture of aluminium powder and ferric oxide in the ratio 1: 3.
ALUMINIUM SULPHATE, Al2(SO4)3
It is used for obtaining H2S in pure form and for making fire proof clothes.
ALLOYS OF ALUMINIUM
|
Alloy |
Composition |
Properties |
Uses |
|
Duralumin |
Light, tough, ductile |
Aeroplanes and automobile parts |
|
|
Aluminium Bronze |
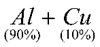 |
Light, strong, golden lustre |
Coins, jewellery |
|
Alcald |
Duralumin coated with pure aluminium |
corrosion resistant, strong |
aircraft industry |
|
Magnalium |
Light, tough and strong |
Balance beams and machinery |
|
|
Alnico |
Highly Magnetic |
Permanent magnets |
ALUMS
The term alum is given to double sulphates of the type X2SO4, Y2(SO4)3. 24H2O where X represents a monovalent cation such as Na+,K+ and NH Rb+, Cs+, Ag+ while Y is a trivalent cation such a Al3+, Cr3+ and Fe3+, Co3+, Ga3+, V3+, Ti3+. (Li+ is too small to be accommodated in the lattice)
General formula :
![]() or
or ![]()
Some important alums are :
· Potash alumK2SO4. Al2(SO4)3. 24H2O
· Sodium alumNa2SO4. Al2(SO4)3. 24H2O
· Ammonium alum(NH4)2SO4. Al2(SO4)3. 24H2O
· Ferric alum(NH4)2SO4. Fe2(SO4)3. 24H2O
· Chrome alumK2SO4. Cr2(SO4)3. 24H2O
Out of these, potash alum is the most important which is prepared in the laboratory by mixing hot solutions of equimolar quantities of K2SO4 and Al2(SO4)3. The resulting solution on concentration and crystallization gives potash alum (emperical formula is KAl(SO4)2.12H2O).
Pseudo alums : When monovalent element of ordinary alums is replaced by a bivalent element eg Mn2+, Fe2+, Mg2+, Cu2+ or Zn2+, the alums are called pseudo alums.
Examples :FeSO4, Al2(SO4)3.24H2O
Ferrous aluminium pseudo alum
MnSO4.Al2(SO4)3.24H2O
Manganese aluminium pseudo alum
PROPERTIES
Potash alum is a white crystalline compound.
The aqueous solution of all alums is acidic due to hydrolysis of Al2(SO4)3, Cr2(SO4)3 or Fe2(SO4)3 as given below
Al2(SO4)3 + 6H2O ![]() 2Al(OH)3 + 3H2SO4
2Al(OH)3 + 3H2SO4
On heating all alums lose water of crystallization
and swell up. The anhydrous alum is known as burnt alum.
Ionisation of aqueous solution of a double salt is as
K2SO4. Al2(SO4)2. 24H2O ![]() K+ + 2Al3+ + 3SO + 24H2O
K+ + 2Al3+ + 3SO + 24H2O
USES
· In purification of water
· For sizing of paper
· As a styptic to stop bleeding
· As a mordant in dyeing and tanning of leather