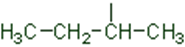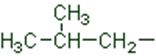CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
CLASSIFICATION OF ORGANIC COMPOUNDS
Organic chemistry is a branch of chemistry that deals with the study of carbon-containing compounds and their properties, reactions, and synthesis. Organic compounds can be found in nature, such as in plants and animals, as well as in man-made products like pharmaceuticals, plastics, and synthetic fibers.
Organic chemistry can be broadly classified into two categories:
· Acyclic or open-chain compounds: These are organic compounds that have a straight or branched chain of carbon atoms. Examples include alkanes, alkenes, alkynes, alcohols, and carboxylic acids.
· Cyclic or closed-chain compounds: These are organic compounds that have a ring or cyclic structure of carbon atoms. Examples include cycloalkanes, aromatic compounds like benzene, and heterocyclic compounds that contain atoms other than carbon in the ring, such as pyridine and furan.
Acyclic or open chain compounds
These compounds contain open chains of carbon atoms in their molecules.The carbon chains may be either straight or branched chains.
(1) Straight chain compounds
n-butane CH3—CH2—CH2—CH3
But-2-ene ![]()
But-2-yne ![]()
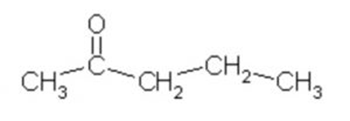
Pentanone
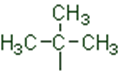
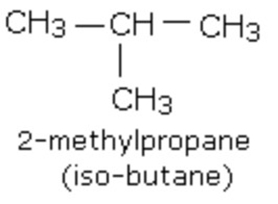
(2) Branched chain compounds
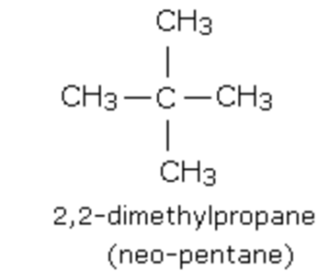
Alicyclic or closed chain or ring compounds
These are the compounds in which the carbon atoms are linked to each other or to the atoms of other elements in such a manner that the molecule has a closed-chain or cyclic or ring structure.
Examples:
|
Structure |
Name |
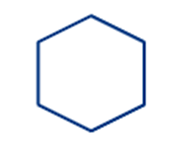 |
Cyclohexane |
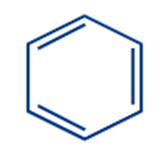 |
Benzene |
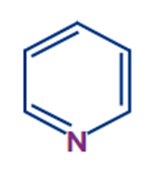 |
Pyridine |
1. Alicyclic (or aliphatic cyclic compounds): An alicyclic compound contains one or more all-carbon rings which may be either saturated or unsaturated, but do not have aromatic character.
Example: Cyclopropane
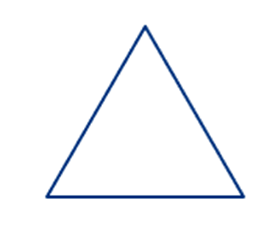
Alicyclic compounds are further classified into homocyclic and heterocyclic compounds:
· Homocyclic Compounds: In homocyclic compounds, all the atoms that form the ring are only carbon atoms.
Example: Cyclohexane
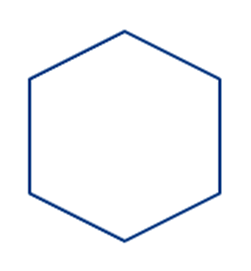
· Aliphatic heterocyclic Compounds: In aliphatic heterocyclic compounds, the cyclic molecule involves carbon as well as heteroatoms like N, O, S, etc.
Example: Tetrahydrothiophene
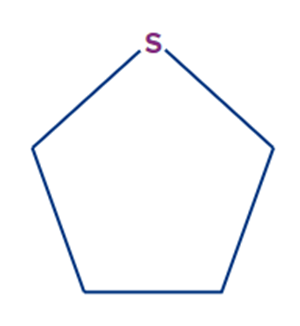
Aromatic compounds
Aromatic compounds are cyclic organic compounds that have a special stability and unique properties due to their electronic structure. These compounds are characterized by having a planar, cyclic, and conjugated structure, and they often have a strong, pleasant odor.
The term "aromatic" was originally used to describe compounds that had a sweet or pleasant aroma, but it is now used to describe a specific type of cyclic compound that meets certain criteria.
The most common example of an aromatic compound is benzene, which has a six-carbon ring with alternating double bonds. The electrons in the double bonds are delocalized, which gives the molecule its characteristic stability and reactivity.
Other examples of aromatic compounds include toluene, naphthalene, and anthracene. Aromatic compounds can also contain functional groups, such as hydroxyl (-OH) or nitro (-NO2) groups, which can affect their properties and reactivity.
Aromatic compounds are widely used in the production of dyes, perfumes, pharmaceuticals, and polymers. They also play important roles in biological processes, such as in the DNA bases adenine and guanine, which are aromatic compounds.
Benzenoid aromatic compounds
The benzenoid aromatic compounds are referred to as those compounds which contain only benzene rings in the whole structure. The benzene ring may be one or more than one. Examples of Benzenoid aromatic compounds are benzene-benzene contains a single ring, naphthalene- naphthalene contains two benzene rings.
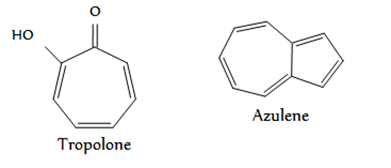
Non-benzenoid compound
There are aromatic compounds, which have structural units different from benzenoid type and are known as Non-benzenoid aromatics e.g. Tropolone, azulene etc.
Heterocyclic aromatic compounds
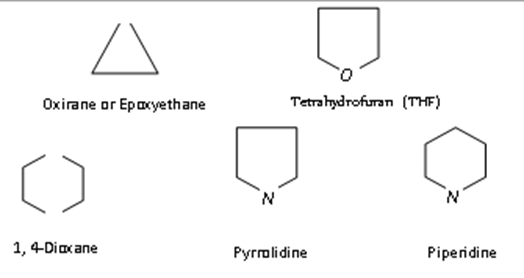
Cyclic compounds containing one or more hetero atoms (e.g. O, N, S etc.) in the ring are called heterocyclic compounds. These are of two types:
Alicyclic heterocyclic compounds:Heterocyclic compounds which resemble aliphatic compounds in their properties are called Alicyclic heterocyclic compounds. For example,
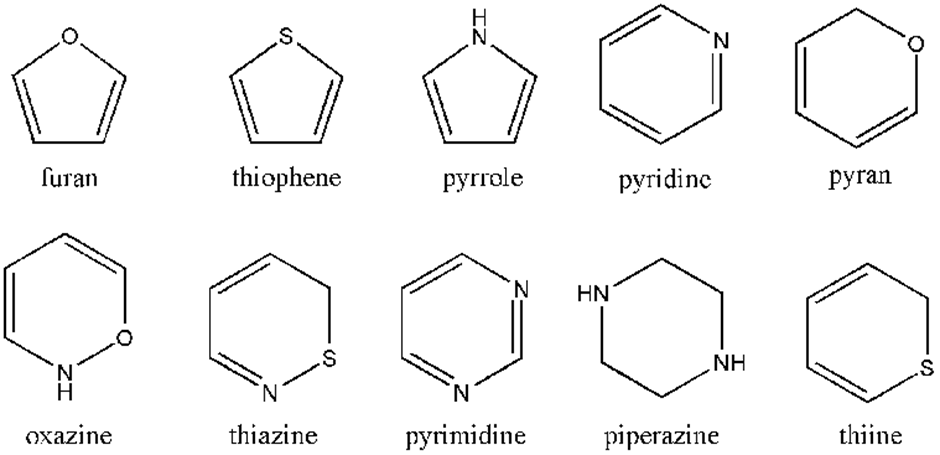
Aromatic heterocyclic compounds:Heterocyclic compounds which resemble benzene and other aromatic compounds in most of their properties are called Aromatic heterocyclic compounds. For example,
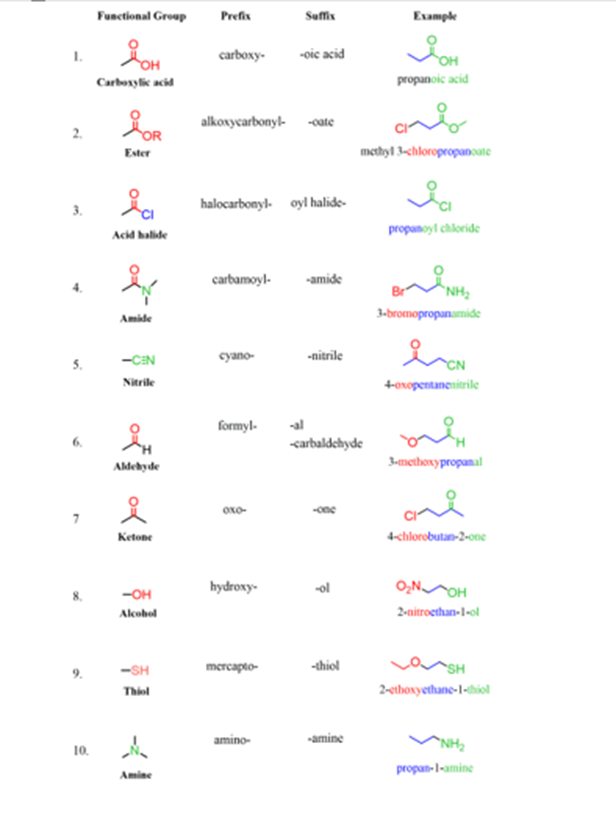
Functional Group
- Functional groups determine the physical and chemical properties of molecules
- The table below shows a summary of common functional groups found in compounds
- R is any other atom or group of atoms (except for hydrogen)
Functional groups found in compounds table
Organic Functional Groups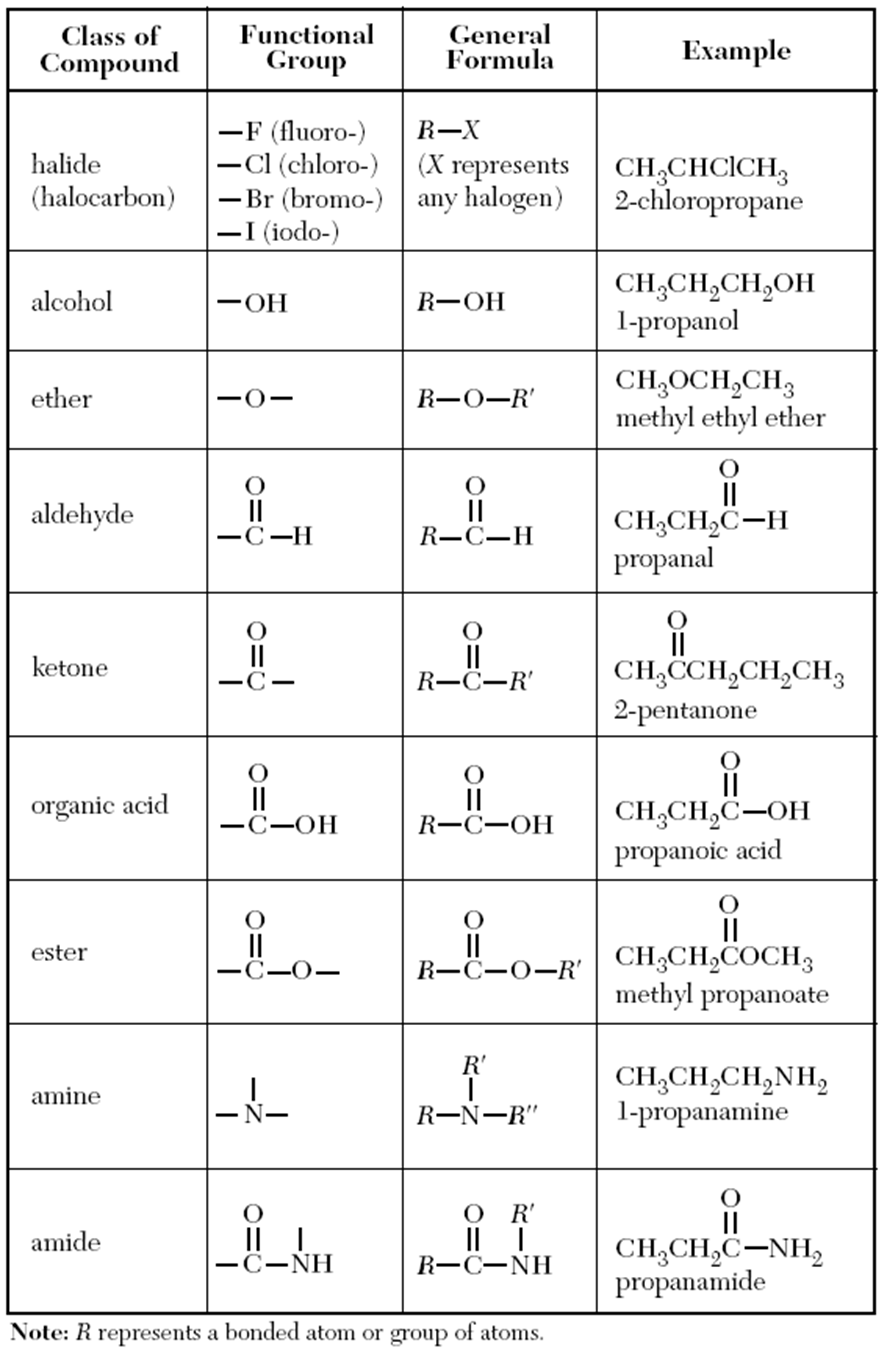
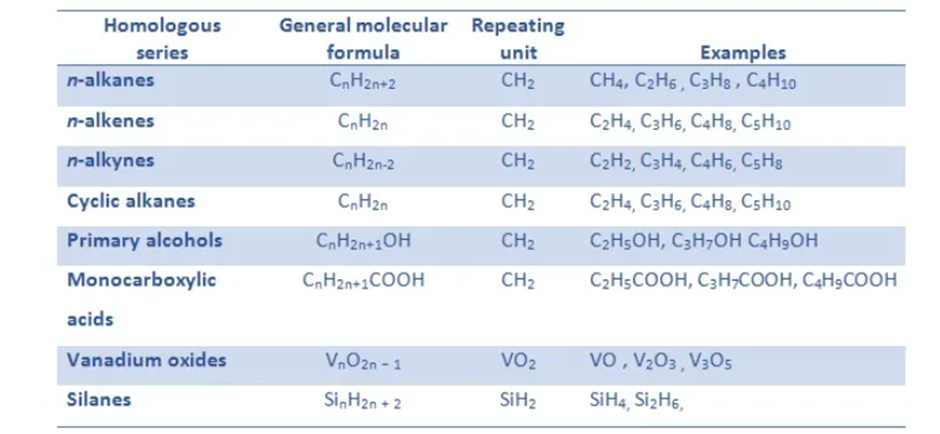
Homologous Series
A group or a series of organic compounds each containing a characteristic functional group forms a homologous series and the members of the series are calledhomologues. The members of a homologous series can be represented by general molecular formula and the successive members differ from each other in molecular formula by a –CH2unit. There are a number of homologous series of organic compounds. Some of these are alkanes, alkenes, alkynes, haloalkanes, alkanols, alkanals, alkanones, alkanoic acids, amines etc.
It is also possible that a compound contains two or more identical or different functional groups. This gives rise to polyfunctional compounds.
NOMENCLATURE OF ORGANIC COMPOUNDS
The IUPAC System of Nomenclature
In the earlier days, the conventional names for organic compounds were mainly derived from the source of occurrence & their properties. However, organic chemists realized the need for a systematic naming for organic compounds since a large number of organic compounds are synthesized in due course. This leads to setting up a system of nomenclature by"International Union of Pure and Applied Chemistry, IUPAC".
TheIUPAC system of nomenclatureis a set of logical rules framed which are mainly aimed at giving an unambiguous name to an organic compound. By using this system, it is possible to give a systematic IUPAC name to an organic compound just by looking at its structure and it is also possible to write the structure of organic compound by following the IUPAC name for that compound.
The systematicIUPAC nameof an organic compound consists of four parts.
1. Root word
2. Suffix(es)
3. Prefix(es)and
4. infix

The suffix is again divided into primary and secondary. Therefore, the complete systematic IUPAC name can be represented as:
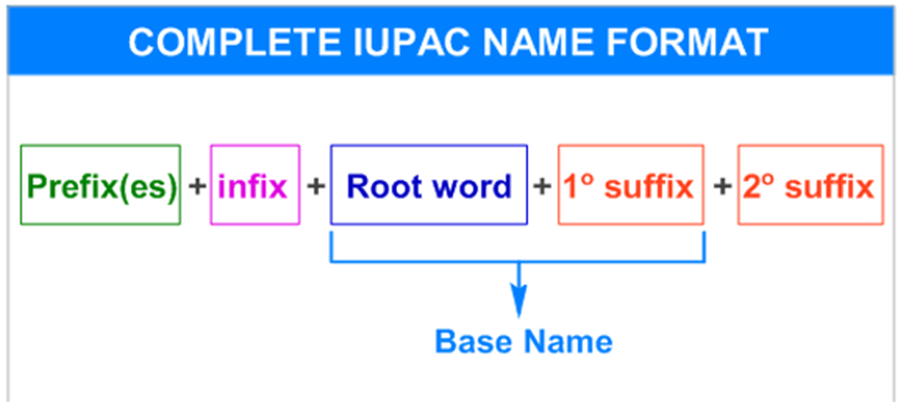
* The"word root"and "1osuffix"together is known asbase name.
* The Prefix(es), infix and 2osuffix may or may not be required always.
The Word root of IUPAC name indicates the number of carbon atoms in the longest possible continuous carbon chain also known asparent chainchosen by a set of rules. The word roots used for different length of carbon chain (upto 20) are shown below.
|
Number of carbon atoms in the parent chain |
Root word |
|
1 |
Meth |
|
2 |
Eth |
|
3 |
Prop |
|
4 |
But |
|
5 |
Pent |
|
6 |
Hex |
|
7 |
Hept |
|
8 |
Oct |
|
9 |
Non |
|
10 |
Dec |
|
11 |
Undec |
|
12 |
Dodec |
|
13 |
Tridec |
|
14 |
Tetradec |
|
15 |
Pentadec |
|
16 |
Hexadec |
|
17 |
Heptadec |
|
18 |
Octadec |
|
19 |
Nonadec |
|
20 |
Icos |
It is again divided into two types.
i. Primary suffix and
ii. Secondary suffix
i) Primary suffix:
It is used to indicate the degree of saturation or unsaturation in the main chain. It is added immediately after the word root of IUPAC name.
|
Type of carbon chain |
Primary suffix |
|
Saturated (all C-C bonds) |
-ane |
|
Unsaturated: one C=C |
-ene |
|
Unsaturated: two C=C |
-diene |
|
Unsaturated: one C≡C |
-yne |
|
Unsaturated: two C≡C |
-diyne |
|
Unsaturated: one C=C & one C≡C |
-enyne |
ii) Secondary suffix:
It is used to indicate the main functional group in the organic compound and is added immediately after the 1osuffix in the IUPAC name.
Note: If there are two or more functional groups in a compound, the functional group with higher priority is to be selected as main functional group, which must be indicated by a secondary suffix. The remaining functional groups with lower priority are treated as substituents and are indicated by prefixes.
The suffixes as well as prefixes used for some important functional groups are shown in the following table in the decreasing order of their priority.
Also note that different suffix is used when carbon atom of the functional group is not part of the main chain.
|
Name of Functional group |
Representation |
Suffix |
Suffix |
Prefix |
|
carboxylic acid |
-COOH |
-oic acid |
-carboxylic acid |
carboxy- |
|
Acid anhydride |
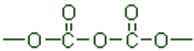 |
-oic anyhydride |
-carboxylic anhydride |
- |
|
Ester |
-COOR |
alkyl -oate |
alkyl -carboxylate |
alkoxycarbonyl- |
|
Acid halide |
-COX |
-oyl halide |
-carbonyl halide |
halocarbonyl- |
|
Acid amide |
-CONH2 |
-amide |
-carboxamide |
carbamoyl- |
|
Nitrile |
-CN |
-nitrile |
-carbonitrile |
cyano- |
|
Aldehyde |
-CHO |
-al |
-carbaldehyde |
oxo- |
|
Ketone |
-CO- |
-one |
- |
oxo- |
|
Alcohol |
-OH |
-ol |
- |
hydroxy |
|
Thiol |
-SH |
-thiol |
- |
mercapto |
|
Amine |
-NH2 |
-amine |
- |
amino- |
|
Imine |
=NH |
-imine |
- |
imino- |
|
Alkene |
C=C |
-ene |
- |
- |
|
Alkyne |
C≡C |
-yne |
- |
- |
The prefix is used to indicate the side chains, substituents and low priority functional groups (which are considered as substituents). The prefix may precede the word root or the infix of IUPAC name.
The prefixes used for some common side chains and substituents are shown below. (the prefixes for functional groups are already given)
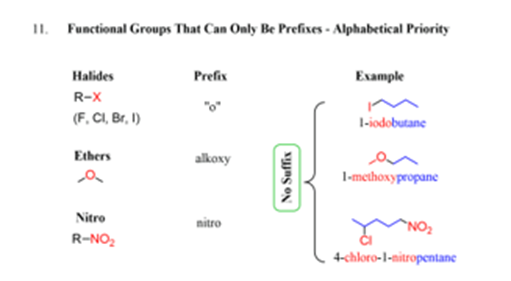
Remember that the alkyl groups along with halo, nitro and alkoxy have the same preference. They have lower priority than double and triple bonds.
The infixes, like cyclo, spiro, bicyclo, are added between the prefix(es) and root word in the IUPAC name to indicate the nature of parent chain.
* The "Cyclo" infix is used to indicate the cyclic nature of the parent chain.
* The "Spiro" infix is used to indicate the spiro compound.
* The "Bicyclo" infix is used to indicate the bicyclic nature of the parent chain.
The infixes are some times called as primary prefixes.
Steps involve in writing IUPAC anme
1) The first step in giving IUPAC name to an organic compound is to select the parent chain and assign a word root.
2) Next, the appropriate primary suffix(es) must be added to the root word to indicate the saturation or unsaturation.
3) If the molecule contains functional group or groups, a secondary suffix must be added to indicate the main functional group. This is optional and not necessary if the molecule containsnofunctional group.
4) Prefix the root word with the infix "cyclo" if the parent chain is cyclic; or with the infix "spiro" if it is a spiro compound; or with the infix "bicyclo" if the compound is bicyclic.
5) Finally add prefix(es) to the IUPAC name, if there are side chains or substituents on the parent chain.
E.g. The IUPAC name of the following compound (3-methylbutan-2-ol) is arrived in steps mentioned below.
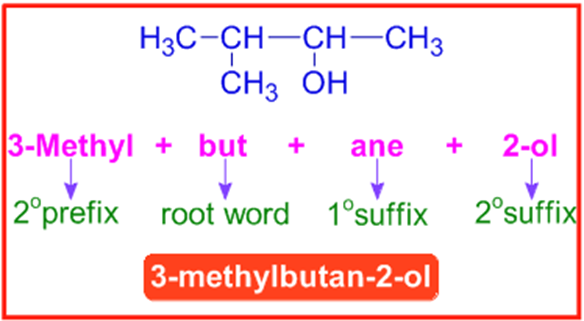
|
Step-1 |
How many carbons are there in the parent chain? |
4 |
Root word = "but" |
|
Step-2 |
Saturated or Unsaturated? |
Saturated |
1osuffix = "ane" |
|
Step-3 |
Is there any functional group? |
Yes. There is an alcohol group on 2nd carbon. |
2osuffix = "2-ol" |
|
Step-4 |
Are there any side chains or substituents? |
Yes. There is a methyl group on 3rd carbon. |
2oprefix = "3-methyl" |
Nomenclature of alkanes
Nomenclature of Straight chain alkanes
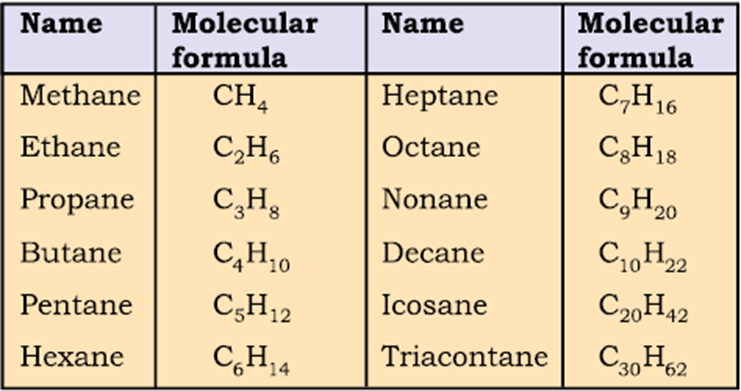
Straight chain hydrocarbons:The names of such compounds are based on their chain structure, and end with suffix ‘-ane’ and carry a prefix indicating the number of carbon atoms present in the chain (except from CH4to C4H10, where the prefixes are derived from trivial names). The IUPAC names of some straight chain saturated hydrocarbons are given in Table . The alkanes in Table differ from each other by merely the number of -CH2groups in the chain. They are homologues of alkane series.
Table IUPAC Names of Some Unbranched Saturated Hydrocarbons
Nomenclature of branched chain alkanes
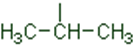
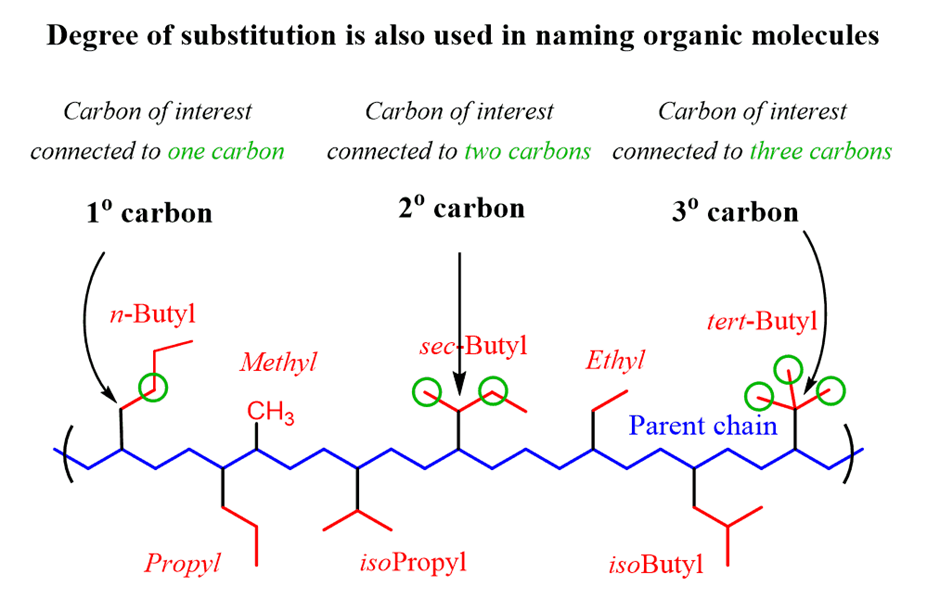
In a branched chain compound small chains of carbon atoms are attached at one or more carbon atoms of the parent chain. The small carbon chains (branches) are called alkyl groups. For example:
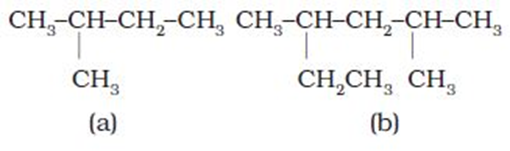
In order to name such compounds, the names of alkyl groups are prefixed to the name of parent alkane. An alkyl group is derived from a saturated hydrocarbon by removing a hydrogen atom from carbon. Thus, CH4becomes -CH3and is calledmethyl group. An alkyl group is named by substituting ‘yl’ for ‘ane’ in the corresponding alkane. Some alkyl groups are listed in Table.
Table Some Alkyl Groups
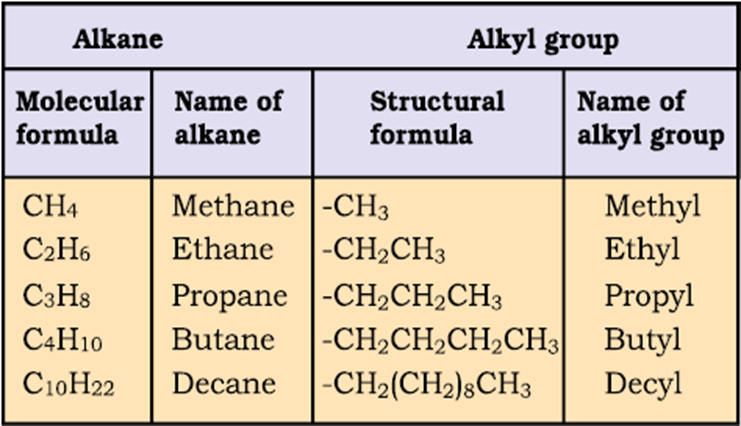
Abbreviations are used for some alkyl groups. For example, methyl is abbreviated as Me, ethyl as Et, propyl as Pr and butyl as Bu. The alkyl groups can be branched also. Thus, propyl and butyl groups can have branched structures as shown below.
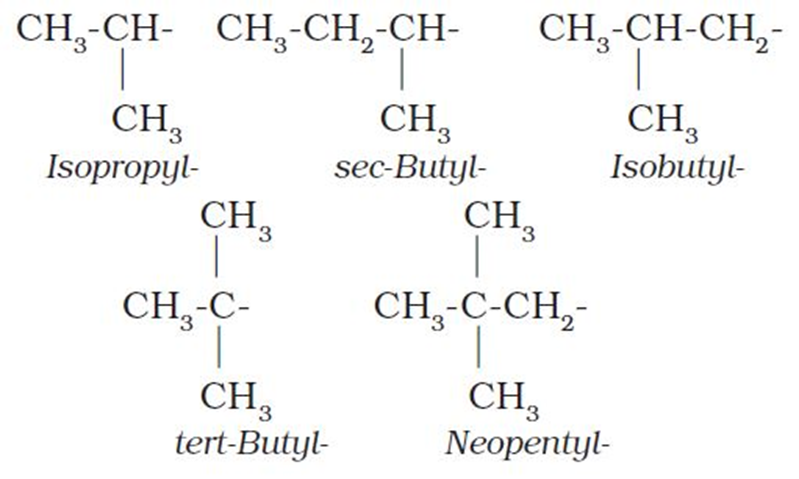
Common branched groups have specific trivial names. For example, the propyl groups can either ben-propyl group or isopropyl group. The branched butyl groups are calledsec-butyl, isobutyl andtert-butyl group. We also encounter the structural unit,–CH2C(CH3)3, which is called neopentyl group.
Nomenclature of branched chain alkanes:We encounter a number of branched chain alkanes. The rules for naming them are given below.
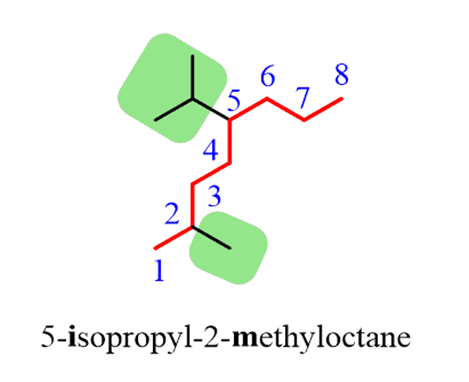
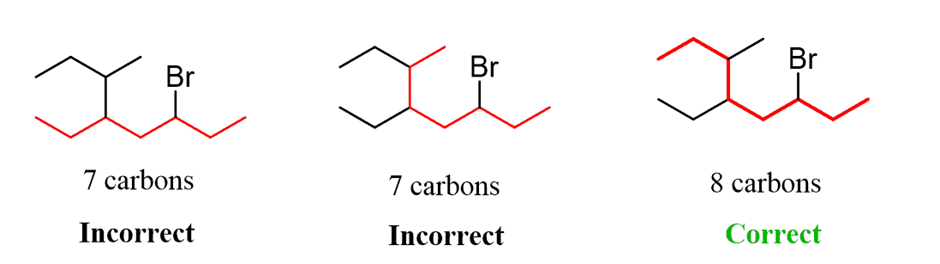
1. First of all, thelongest carbon chain in the molecule is identified. In the example (I) given below, the longest chain has nine carbons and it is considered as theparentorrootchain. Selection of parent chain as shown in (II) is not correct because it has only eight carbons.
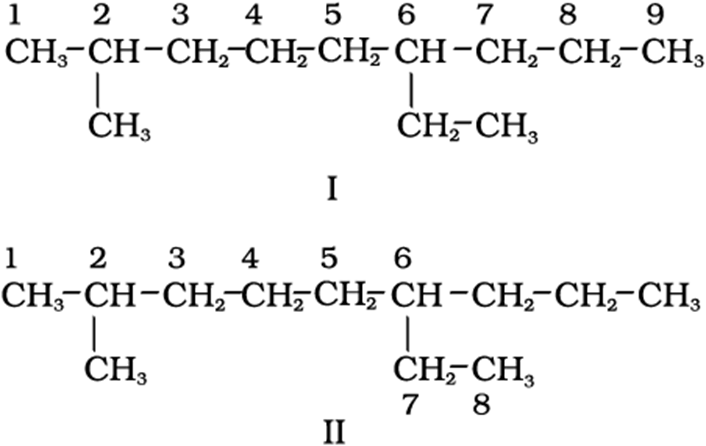
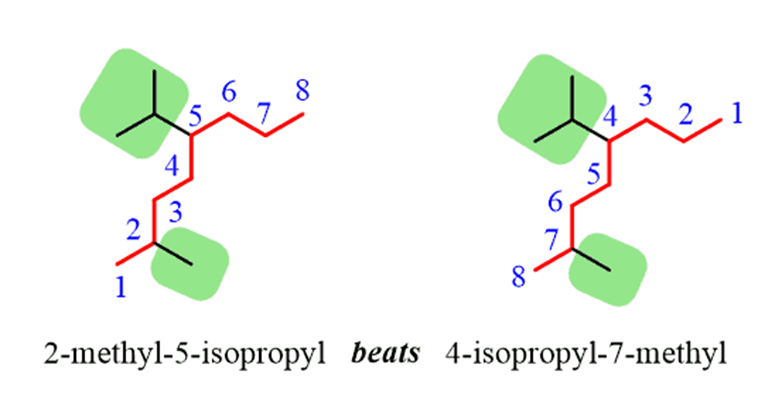
2.The carbon atoms of the parent chain are numbered to identify the parent alkane and to locate the positions of the carbon atoms at which branching takes place due to the substitution of alkyl group in place of hydrogen atoms.The numbering is done in such a way that the branched carbon atoms get the lowest possible numbers.Thus, the numbering in the above example should be from left to right (branching at carbon atoms 2 and 6) and not from right to left (giving numbers 4 and 8 to the carbon atoms at which branches are attached).
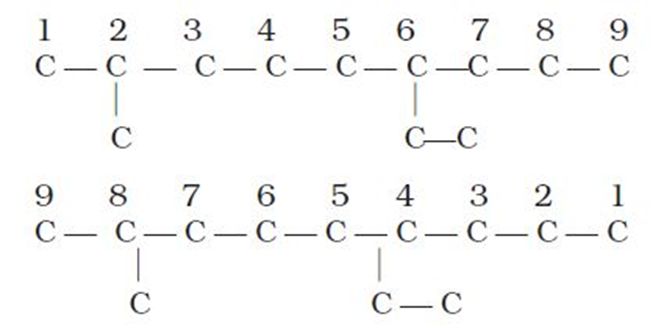
3. The names of alkylgroups attached as a branch are then prefixed to the name of the parent alkaneand position of the substituents is indicated by the appropriate numbers. If different alkyl groups are present, they are listed in alphabetical order. Thus, name for the compound shown above is: 6-ethyl-2-methylnonane. [Note: the numbers are separated from the groups by hyphens and there is no break between methyl and nonane.]
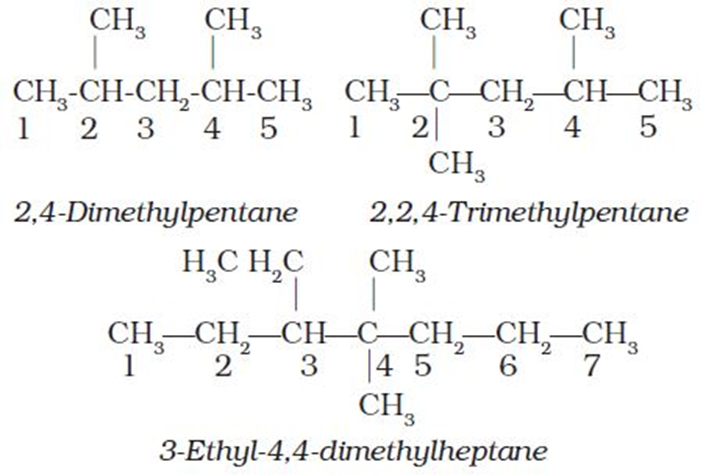
4. If two or more identical substituent groups are present then the numbers are separated by commas. The names of identical substituents are not repeated, instead prefixes such as di (for 2), tri(for 3), tetra (for 4), penta (for 5), hexa (for 6) etc. are used. While writing the name of the substituents in alphabetical order, these prefixes, however, are not considered. Thus, the following compounds are named as:
5. If the two substituents are found in equivalent positions, thelower number is given to the one coming first in the alphabetical listing. Thus, the following compound is 3-ethyl-6-methyloctane and not 6-ethyl-3-methyloctane.
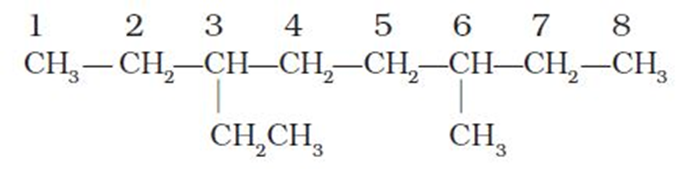
6. The branched alkyl groups can be named by following the above mentioned procedures. However, thecarbon atom of the branch that attaches to the root alkaneis numbered 1 as exemplified below.
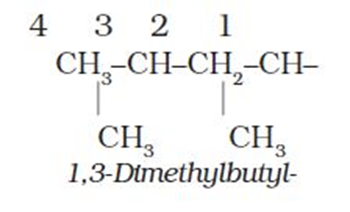
The name of such branched chain alkyl group is placed in parenthesis while naming the compound. While writing the trivial names of substituents’in alphabetical order, the prefixes iso- and neo- are considered to be the part of the fundamental name of alkyl group. The prefixessec- andtert- are notconsidered to be the part of the fundamental name. The use ofisoand related common prefixes for naming alkyl groups is also allowed by the IUPAC nomenclature as long as these are not further substituted. In multi-substituted compounds, the following rules may aso be remembered:
• If there happens to be two chains of equal size, then that chain is to be selected which contains more number of side chains.
• After selection of the chain, numbering is to be done from the end closer to the substituent.
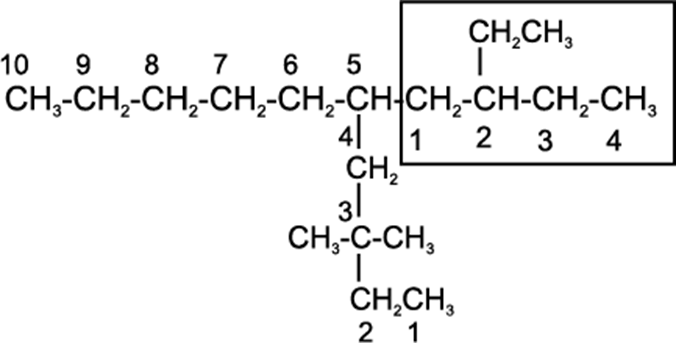
5-(2-Ethylbutyl)-3,3-dimethyldecane
[and not 5-(2,2-Dimethylbutyl)-3-ethyldecane]
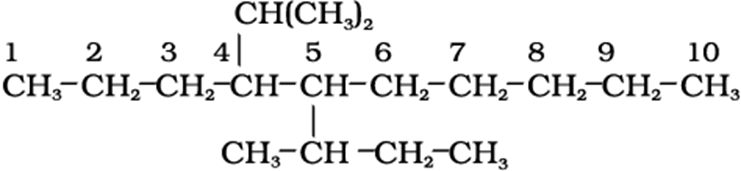
5-sec-Butyl-4-isopropyldecane
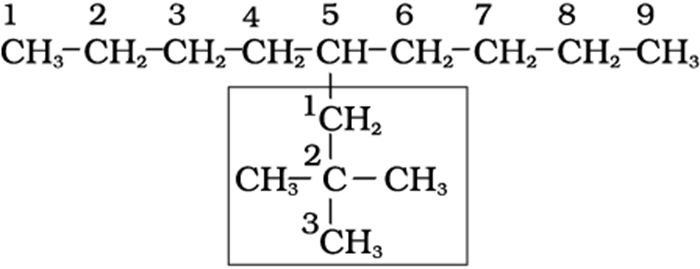
5-(2,2-Dimethylpropyl)nonane
Nomenclature of Organic compounds having functional groups
A functional group, as defined earlier, is an atom or a group of atoms bonded together in a unique manner which is usually the site of chemical reactivity in an organic molecule. Compounds having the same functional group undergo similar reactions. For example, CH3OH, CH3CH2OH, and (CH3)2CHOH — all having -OH functional group liberate hydrogen on reaction with sodium metal. The presence of functional groups enables systematisation of organic compounds into different classes. Examples of some functional groups with their prefixes and suffixes along with some examples of organic compounds possessing these are given in Table .
First of all, the functional group presentin the molecule is identified which determines the choice of appropriate suffix.Thelongest chain of carbon atoms containing the functional group isnumbered in such a way that the functional group is attached at thecarbon atom possessing lowest possible number in the chain.By using the suffix as given in Table , the name of the compound is arrived at.
Inthe case of polyfunctional compounds, one of the functional groups is chosen as theprincipal functional groupand the compound is then named on that basis.Theremainingfunctional groups, which are subordinate functional groups,are named as substituents using the appropriate prefixes.The choice of principal functional group is made on the basis of order of preference.The order of decreasing priority for some functional groups is:
-COOH,–SO3H,-COOR (R=alkyl group), COCl,-CONH2, -CN,-HC=O, >C=O, -OH, -NH2, >C=C<, -C≡C- .
The –R, C6H5-, halogens (F, Cl, Br, I), –NO2, alkoxy (–OR) etc. are always prefix substituents. Thus, a compound containing both an alcohol and a keto group is named as hydroxyalkanone since the keto groupis preferred to the hydroxyl group.
For example, HOCH2(CH2)3CH2COCH3will be named as 7-hydroxyheptan-2-one and not as 2-oxoheptan -7-ol. Similarly, BrCH2CH=CH2is named as 3-bromoprop-1-ene and not 1-bromoprop-2-ene.
If more than one functional group of the same type are present, their number is indicated by adding di, tri, etc. before the class suffix. In such cases the full name of the parent alkane is written before the class suffix. For example CH2(OH)CH2(OH) is named as ethane–1,2–diol. However, the ending – ne of the parent alkane is dropped in the case of compounds having more than one double or triple bond; for example, CH2=CH-CH=CH2is named as buta–1,3–diene.
Table Some Functional Groups and Classes of Organic Compounds
Nomenclature of alkene
Alkenes are normally named using the IUPAC system. The rules for alkenes are similar to those used for alkanes. The following rules summarize alkene nomenclature.
1. Identify the longest continuous chain of carbon atoms that contains the carbon‐carbon double bond.The parent name of the alkene comes from the IUPAC name for the alkane with the same number of carbon atoms, except the‐aneending is changed to‐eneto signify the presence of a double bond. For example, if the longest continuous chain of carbon atoms containing a double bond has five carbon atoms, the compound is a pentene.
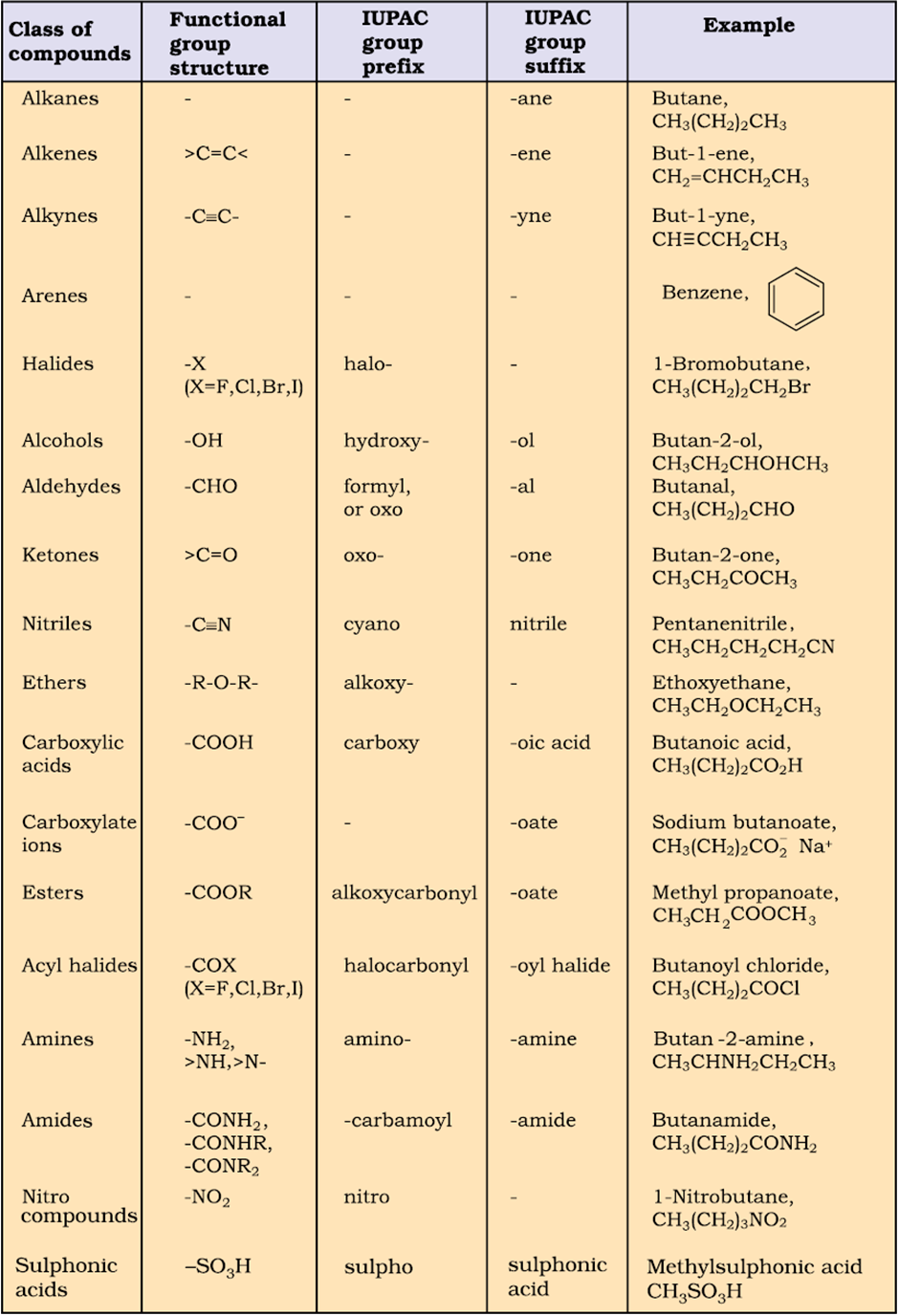
2. Number the carbon atoms of the longest continuous chain, starting at the end closest to the double bond. Thus, is numbered from right to left, placing the double bond between the second and third carbon atoms of the chain. (Numbering the chain from left to right incorrectly places the double bond between the third and fourth carbons of the chain.)
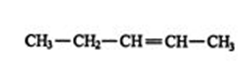
3. The position of the double bond is indicated by placing the lower of the pair of numbers assigned to the double‐bonded carbon atoms in front of the name of the alkene. Thus, the compound shown in rule 2 is 2‐pentene.
4. The location and name of any substituent molecule or group is indicated. For example, is 5‐chloro‐2‐` hexene.
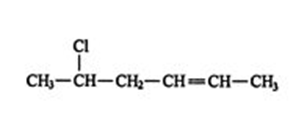
5. Finally, if the correct three‐dimensional relationship is known about the groups attached to the double‐ bonded carbons, thecisortransconformation label may be assigned. Thus, the complete name of the compound in rule 4 (shown differently here) iscis‐5‐chloro‐2‐hexene.
Nomenclature of alkyne
IUPAC NAMING OF ALKYNES
Following rules are used for finding IUPAC names of the alkynes.
1. Select the longest hydrocarbon chain, which is termed as parent/ root or base chain. In the case of alkynes, the chain which is having triple bond will be considered a Root or parent, or base chain. The name of the parent chain is termed with the help of IUPAC.
2. Start numbering the carbons in the longest carbon chain, in alkynes numbering will start from that end to which the triple bond is closest.
3. 1-alkyne will be referred to as terminal alkynes and other triple bonds present in the compound will be referred to as terminal alkynes.
4. After numbering, the next step is naming the compound, remembering that naming should be done according to alphabetical order.
5. For more than one same substituent group present in the same compound we have to use prefixes like di, tri, tetra, etc. For two, three, and four respectively.
6. Substituent groups that have triple bonds are known as alkynyl.
7. Compounds that contain both alkene and alkyne groups are known as alkyne. Numbering will start with a functional group that is closest to the terminal.
8. If both alkene and alkyne are at the same distance then, preference will be given to alkene.
Examples
Write the IUPAC name of the following structures of compounds.
1.
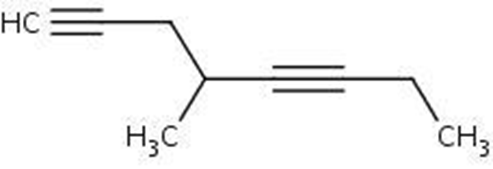
IUPAC Name : 4-methyl-1,5-octadiyne
2. 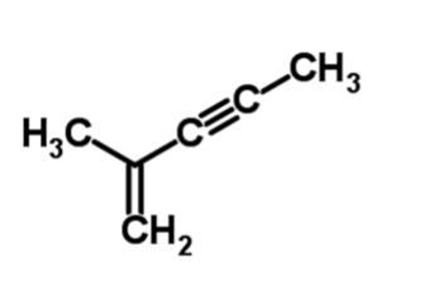
IUPAC Name : 2-methyl-1-pentene-3-yne
3. 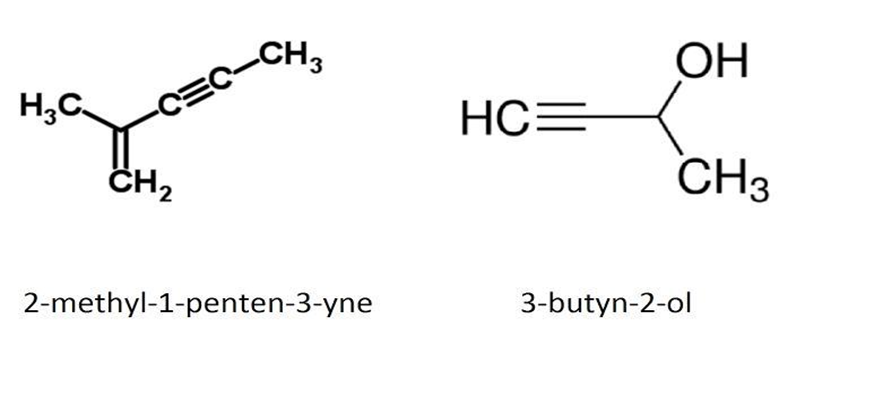 IUPAC Name : 3-butyn-2-ol
IUPAC Name : 3-butyn-2-ol
4. 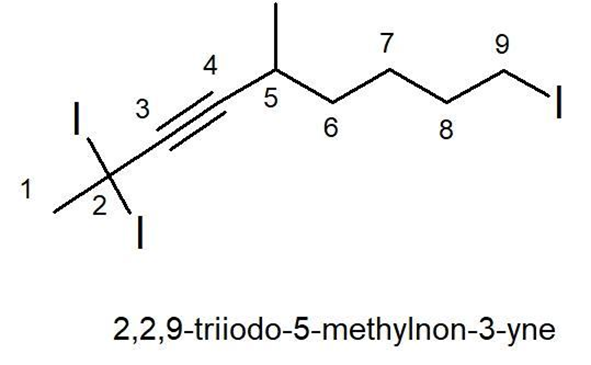
IUPAC Name : 2,2,9-triiodo-5-methyl-non-3-yne
5.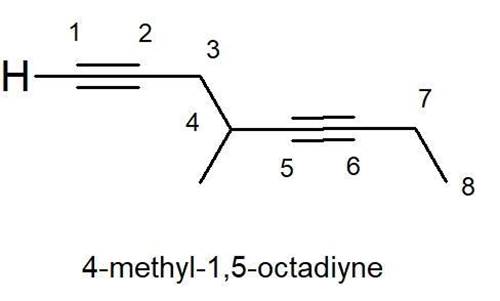
IUPAC Name : 4-methyl-1,5-octadiyne
Nomenclature of cycloalkane
1. Identify the parent chain
- The parent chain is the chain with the cyclic structure. If there is a longer alkyl substituent attached, then the cyclic structure acts as the substituent.
- Count the number of single-bonded carbons in the structure and then place ‘cyclo’ in front of it. (Remember: 3 carbons= propane, 4=butane, 5=pentane, 6=hexane )
- In our example above, there are six carbons. This means the parent chain iscyclohexane.
2. Number the parent chain
- Now that you know the parent chain, you must number it. There are 2 main rules for determining which direction to go:
- Rule 1: Substituents should be placed on the lowest numbers possible. This means 1-methyl is better than 3-methyl.
- Rule 2: If a tie occurs in rule one, choose priority based on the alphabet.For example, if there was one methyl substituent and one ethyl substituent, ethyl would win priority.
- In our example above, we would place a 1 at the ethyl and go counterclockwise. This means we have the following attachments: 1-ethyl, 2-methyl, and 4-methyl.
3. Identify the substituents and how many there are of each.
- Substituents are attachments. This can be carbon chains like methyl groups, branched groups like sec-butyl, orfunctional group. If there are multiple, we will place a numerical prefix in front of it.
- Since we have 2 methyl groups, we will place a “di” in front.
- Moreover, we can group the two methyl groups together. Meaning it will be written as 2,4-dimethyl.
4. Put everything together.
- Substituents should be placed in alphabetical order regardless of the numerical prefixes like “di”.
- The final product for our example would be 1-ethyl-2,4-dimethylcyclohexane.
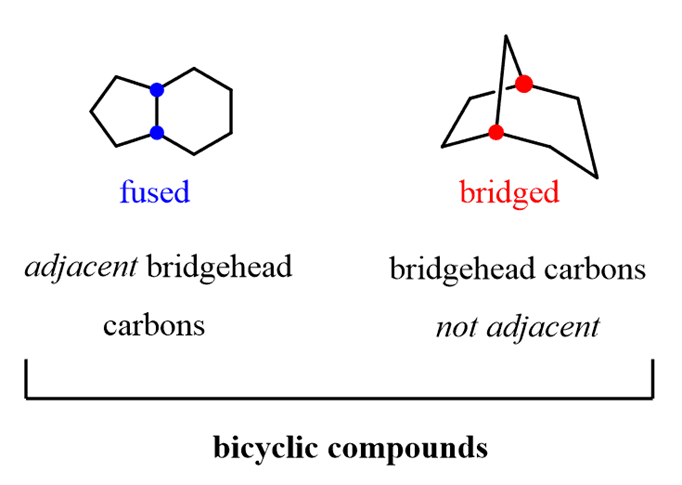
Nomenclature of bicyclic compounds
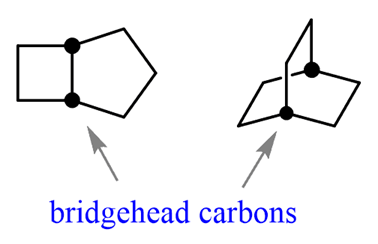
Bicyclic compounds are a specific type ofpolycyclic compounds,classified as compounds that contain more than one ring with at least two common atoms. The common atoms connecting the rings in a bicyclic compound are calledbridgehead carbons:
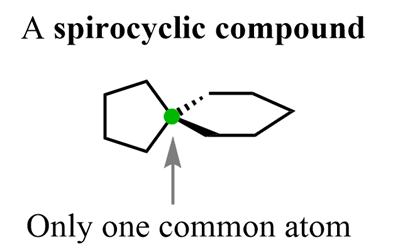
So, in bridged bicyclic compounds, the joint carbons are farther apart and when they get next to each other (connected) we have fused bicyclo compounds. The extreme end of this relationship is when there is only one common carbon, and these are calledspirocyclic compound:
Naming Bicyclic Compounds
The first rule for naming bicyclic compounds is to start numbering from one of the bridgehead carbon atoms. Next, you need to decide the correct direction. For example, what is the correct direction for numbering in the following compound?
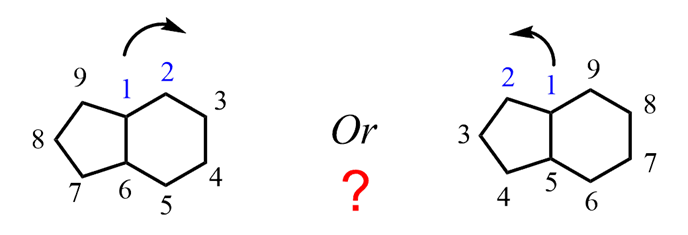
For this, you need to remember that the rings are numbered according to their size – the one with more carbon atoms is numbered first, then the second and etc. So here, we will go clockwise numbering the ring on the right and the one on the left:
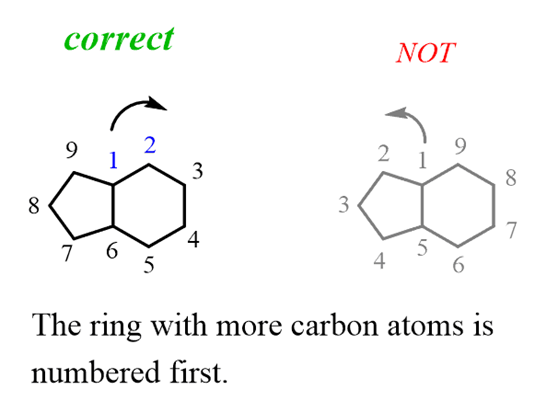
You may wonder why I call them “a ring on the right” and “a ring on the left” instead of cyclohexane and a cyclopentane and the reason is that they are not actually two ringsof cyclohexane and cyclopentane but rather awhole structure with 9 carbon atoms. In fact, theparent chain of bicyclic compoundsis given based on thetotal number of carbonsin all the rings. So, this compound is named as nonane and the number of carbon atoms in each ring is designated as 4 and 3 rather than 6 and 5.
With this said, let’s put together the basic unit for naming bicyclic compounds. It is the word “bicyclo” followed by a square bracket with the number of carbon atoms in each ring:
Bicyclo[#C, #C, #C]parent chain
In the example above, we’d havebicyclo[4.3.0]nonane:
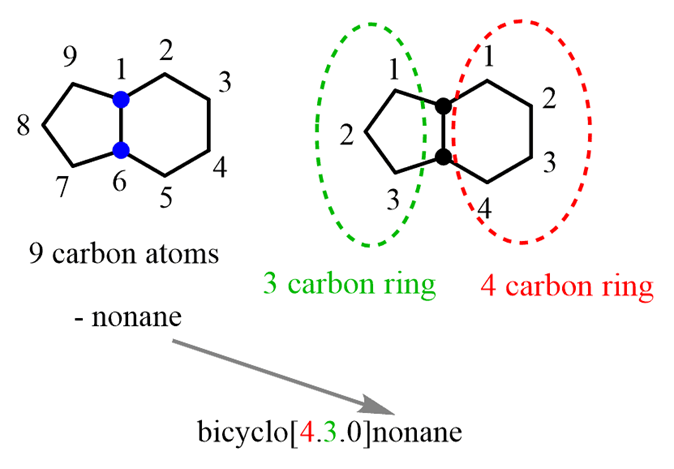
Notice again that the numbers in brackets are showing the number of carbon atoms in each ring excluding the bridgehead carbons.
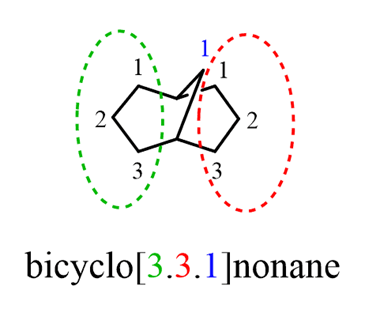
One question you may wonder is thezero in the brackets. This is to specify that there are only two rings and not three (zero carbons in the third ring). For example, we could have another bicyclic nonane with three joint rings:
Nomenclature of compounds containing terminating functional group
Nomenclature of alkyl halides
The nomenclature of alkyl halides follows the same IUPAC rules that we discussed fornaming alkane. The only difference here is that one (or more) of the substituents is a halogen and its naming is a little modified.
Let’s first recall the nomenclature rules by naming the following alkane:
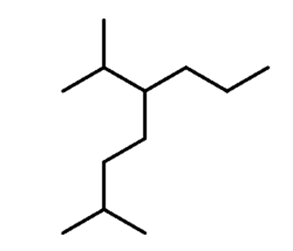
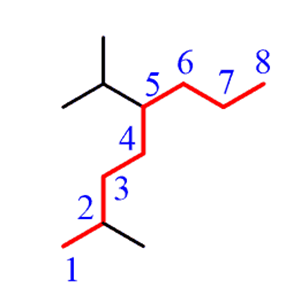
If there are two chains of equal length, thenyou need tochoose the one with the greater number of substituents.
Step 2.Find the substituents. In this case, we have a methyl and an isopropyl group.
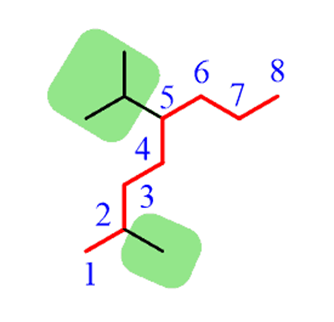
Step 4.Put the parent chain and substituents together byplacing the substituents in alphabetical order!
It is worth mentioning that it is only the “iso” prefix that is considered for alphabetical priority. All the other common groups shown below go based on the first letter of the carbon chain and not the prefix:
Naming Alkyl Halides
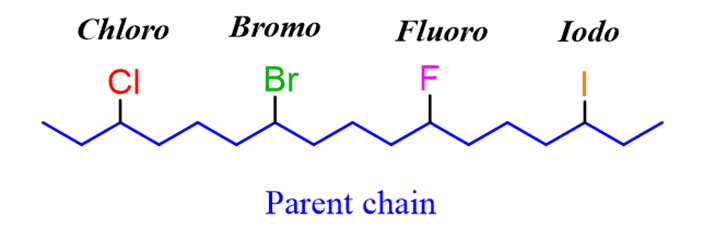
The halogen in alkyl halides is treated just like any alkyl substituent, meaning it has no priority over the carbon atoms. The parent chain s still numbered in a way to give the lowest possible number(s) for the substituents.
The only difference in naming alkyl halides is the change of the suffix “ine” to “o”. For example, 2-bromo, 4-chloro, 3-iodo, 5-fluoro:
Let’s name the following alkyl halide:
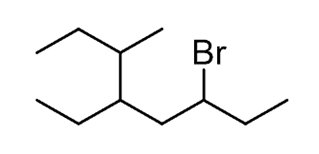
Step 2.Find the substituents. Here, we have three substituents – two alkyl groups and a halide:
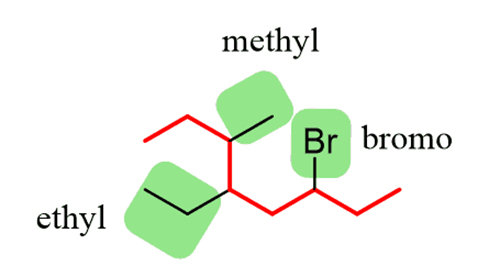
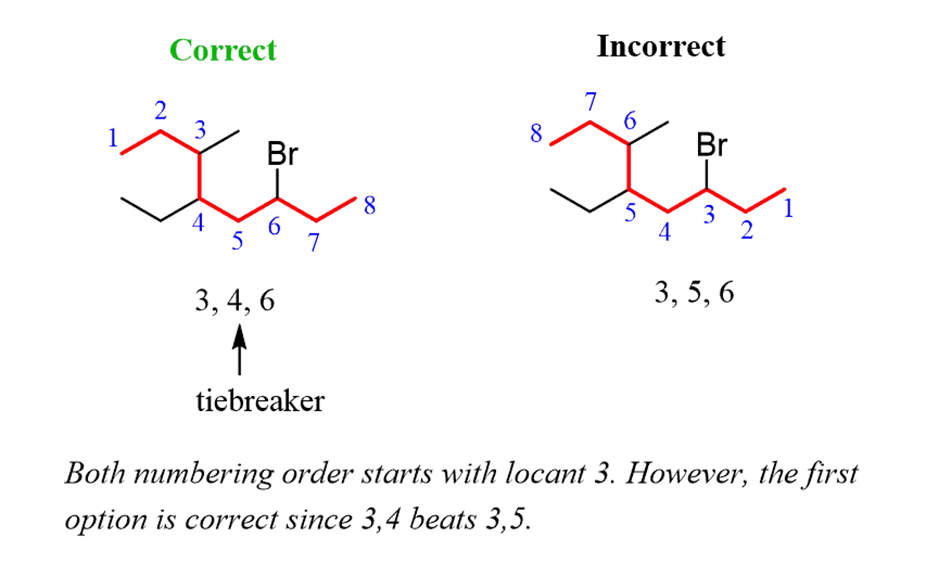
Step 4.Put the parent chain and substituents together byplacing the substituents in alphabetical order:
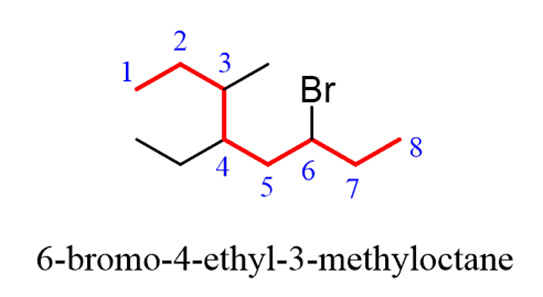
Notice again, that in the final name, the groups are placed in alphabetical order. Even though Br is on position 6, it is still place before the other substituents.
Nomenclature of alcohols
The following procedure should be followed in giving alcohol IUPAC substitutive names.
1. Select the longest continuous chain to which the hydroxyl group is directly attached. Change the name of the alkane corresponding to the chain by dropping the final -e and adding the suffix -ol.
2. Number the longest continuous carbon chain so as to give the carbon atom bearing the hydroxyl group the lower number. Indicate the position of the hydroxyl group by using this number as a locant.
3. Indicate the position of another substituent as a prefix by using the numbers corresponding to their positions along the carbon chain as locants.
The following example shows how the rules are applied.
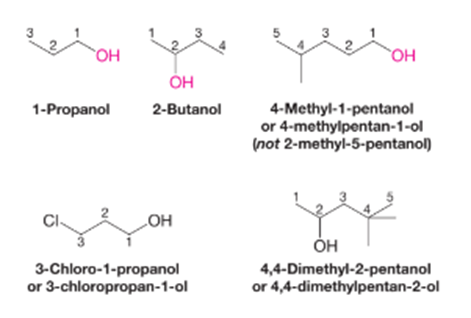
IUPAC Nomenclature for Alcohols
Types of Nomenclature in Alcohols
There are three systems of naming alcohols
1. Common or trivial system
2. Carbinol system and
3. IUPAC system
|
Formula |
Parent Hydrocarbon |
Common name |
Carbinol name |
IUPAC name |
|
CH3-OH |
Methane |
Methyl alcohol |
Carbinol |
Methanol |
|
CH3-CH2-OH |
Ethane |
Ethyl alcohol |
Methyl carbinol |
Ethanol |
|
CH3-CH(OH)-CH3 |
Propane |
Isopropyl alcohol |
Dimethyl carbinol |
2-Propanol |
|
(CH3)3-C-OH |
2-Methyl propane |
Tert-butyl alcohol |
Trimethyl carbinol |
2-Methyl-2-propanol |
Nomenclature of ethers
Ethers are named by both common and systematic nomenclature of the IUPAC rules. The common names are used for ethers with simple alkyl groups. To do this, wefirst identify the alkyl groupsand arrange them inalphabetical orderfollowed by the word“ether”.
For example,
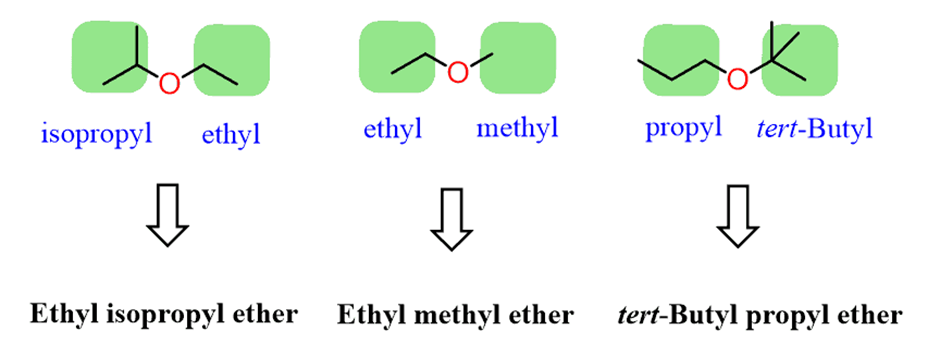
If the groups areidentical – symmetrical ethers, theprefix “di”is added. For example,
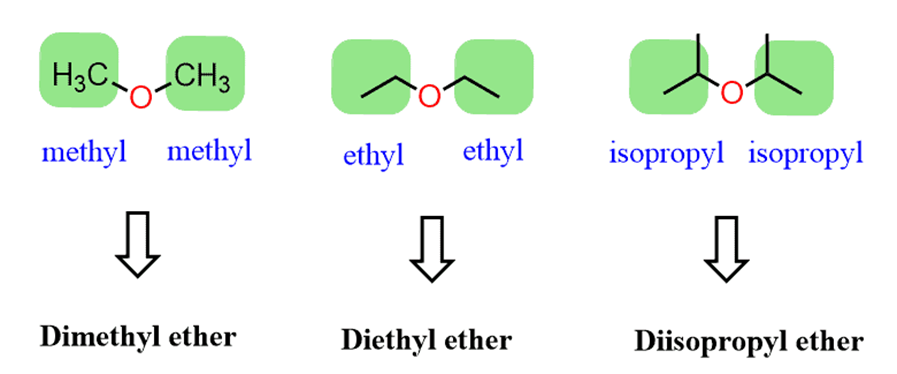
The systematic nomenclature is used for ethers withcomplex substituents. The idea here is to treat one of the alkoxy (alkyl with the oxygen) groups as asubstituentconnected to aparent chain. Theparent chainis determined just like we always do, based on thelongest carbon chain.
For example,
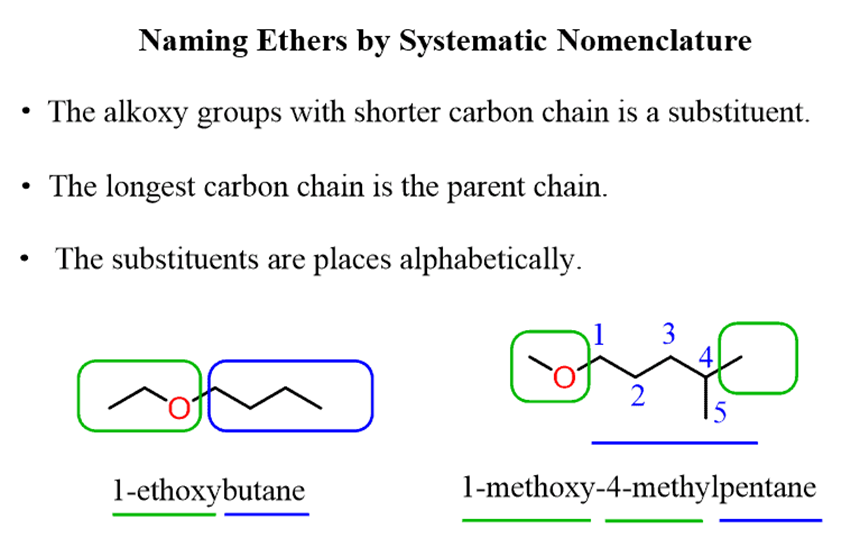
For example, let’s compare the effects of the OH and OR groups on naming structurally similar compounds:
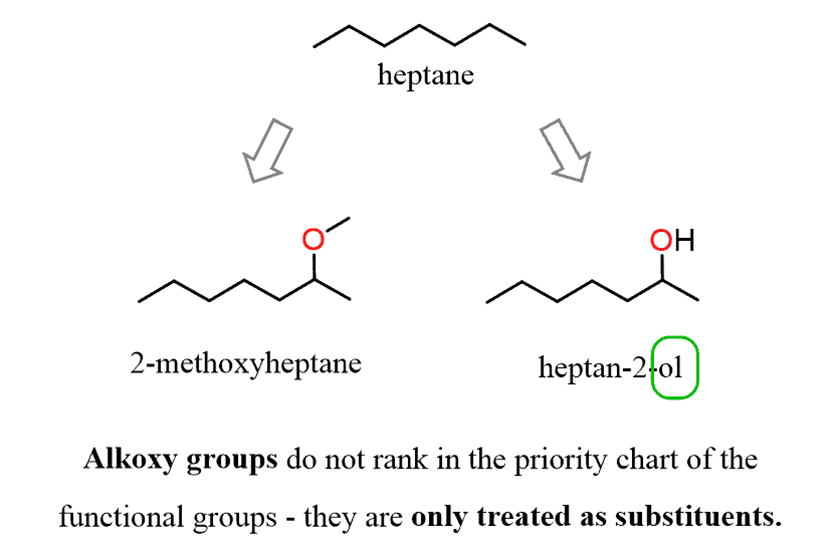
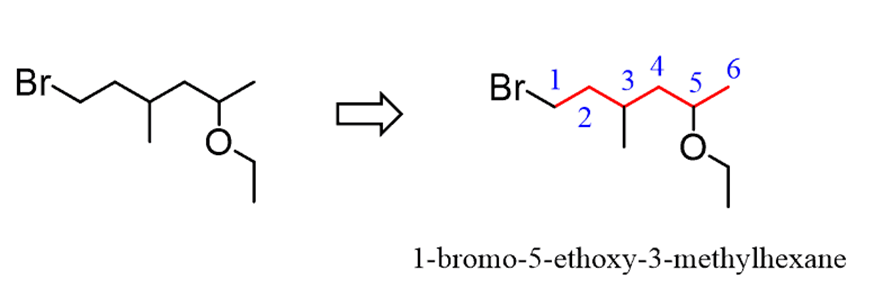
Now, considering this, let’s name the following ether with alkyl and halide substituents:
Nomenclature of amines
Amines
Before going into the details of naming amines, let’s first recall what they are and how they are classified.
Amines are the derivatives ofammonia(remember NH3from General chemistry). Replacing one hydrogen of ammonia with an alkyl group forms an amine with a general formula of R-NH2:
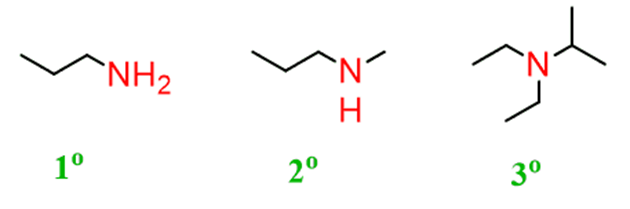
In general, amines can be named either bysystematicorcommonnames.
Naming amines by the systematic nomenclature follows the same rules we discussed earlier for the IUPACnomenclature rules for alkanes.
This is the brief summary of naming a primary amine:
Step 1.Identify the longest carbon chain bonded to the amine nitrogen.
Step 2.Identify the substituents.
Step 3.Number the parent chain giving the amine the lowest locant
Step 4.Put everything together having the substituentsin alphabetical order.
For example, butanechanges to butan-1-amine,cyclohexaneto cyclohexanamine:
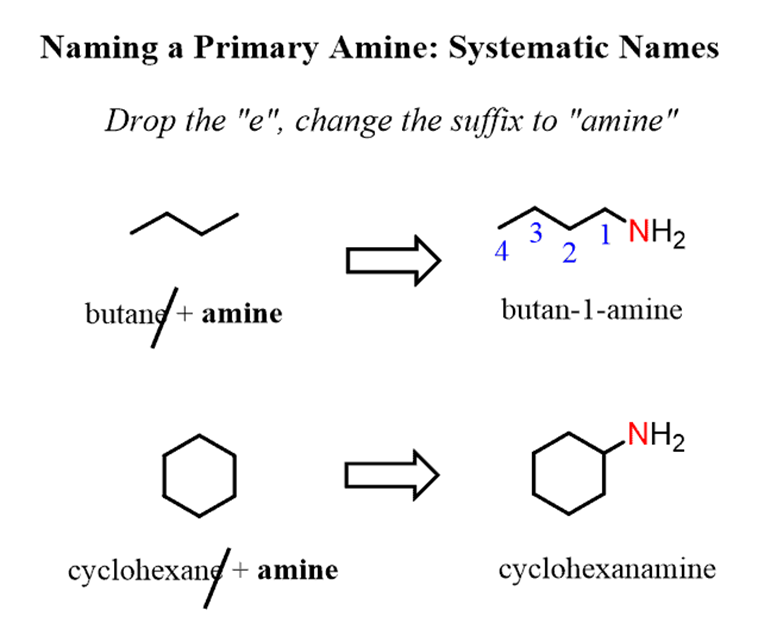
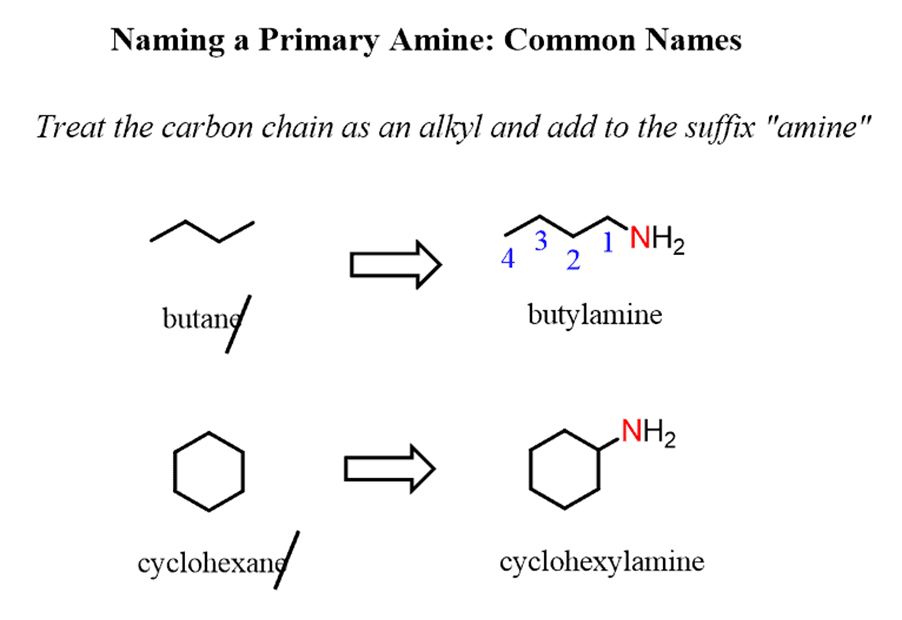
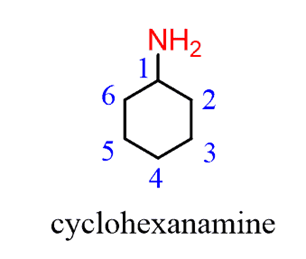
Notice that when theamine is connected to a ring, we start numbering fromthe carbon connected to the NH2group. This rule always puts the NH2group at C1, therefore, the “1” is usually omitted from the name:
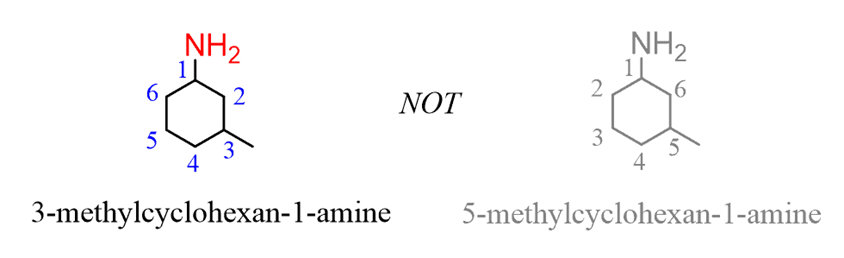
Let’s consider a few more examples demonstrating the priority of the amino group over other functional groups such as alkyls, halides, and multiple bonds. The list of functional group priorities can be found in this post:
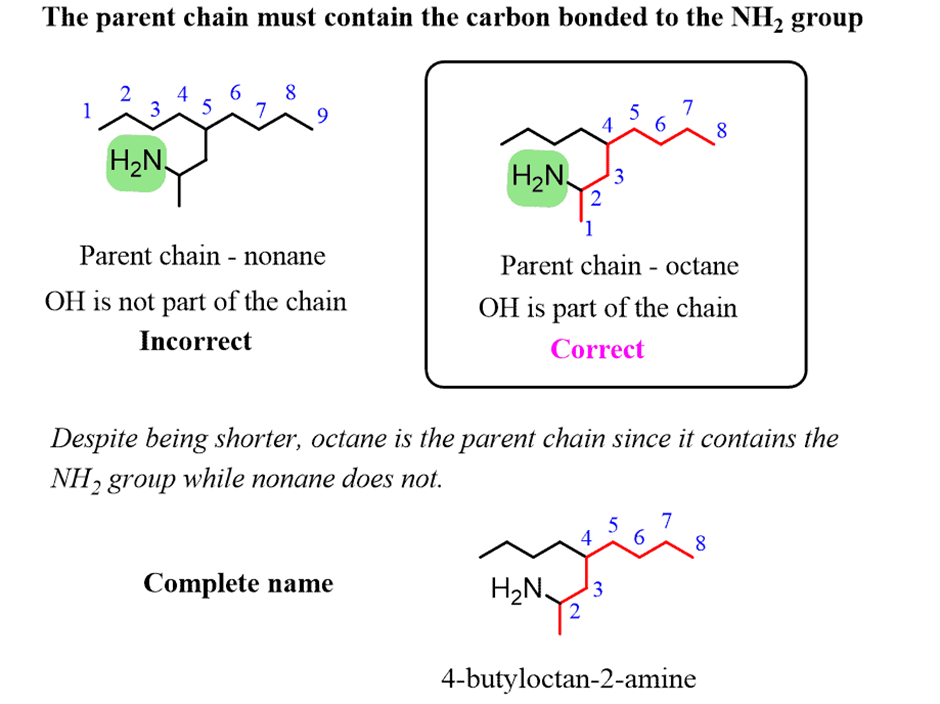
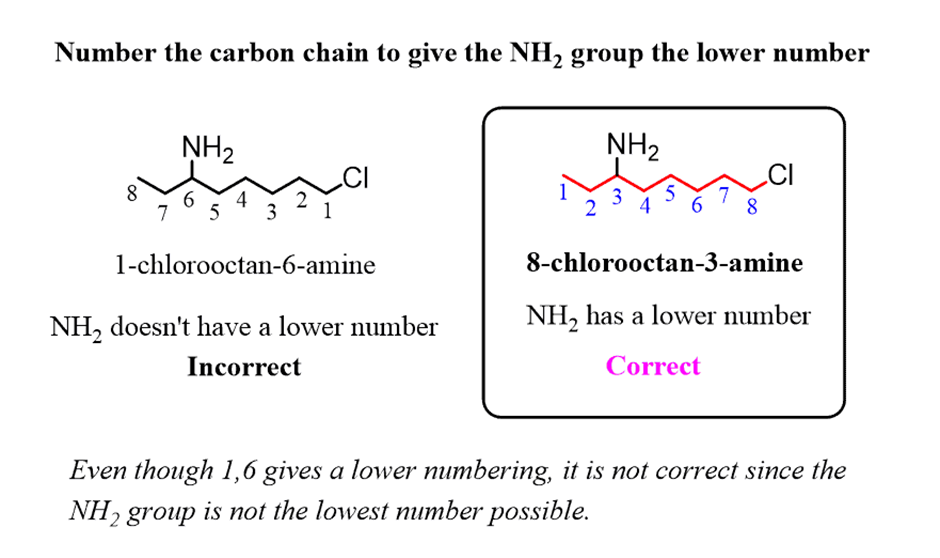
How to Name a Compound with Multiple Functional Groups
Theparent chainis chosen such that it is thelongest carbon chain containingthe carbon atom connected to the NH2groupeven if there is a longer chain without the NH2group:
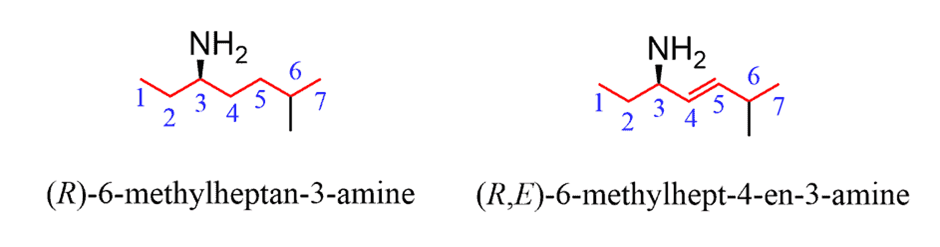
If theNH2group is connected to a chiral center, you will also need to include theabsolute configurationat the beginning of the name. Also, if there is a double, theE and Z configurationshould be addressed when applicable:
Naming a Compound Where the Amino group is Not the Highest Priority
When the compound contains a functional group(s) that havehigher priority than the amino group, then it is represented by aprefix “amino”.
For example, if we put analcohol and amineon the periphery of a carbon chain, thealcohol gets the priority, therefore it is assigned with asuffix, while theamine is assigned a prefix(like the alkyl substitutes). This also indicates that we need to start numbering the carbon chain from the OH group:
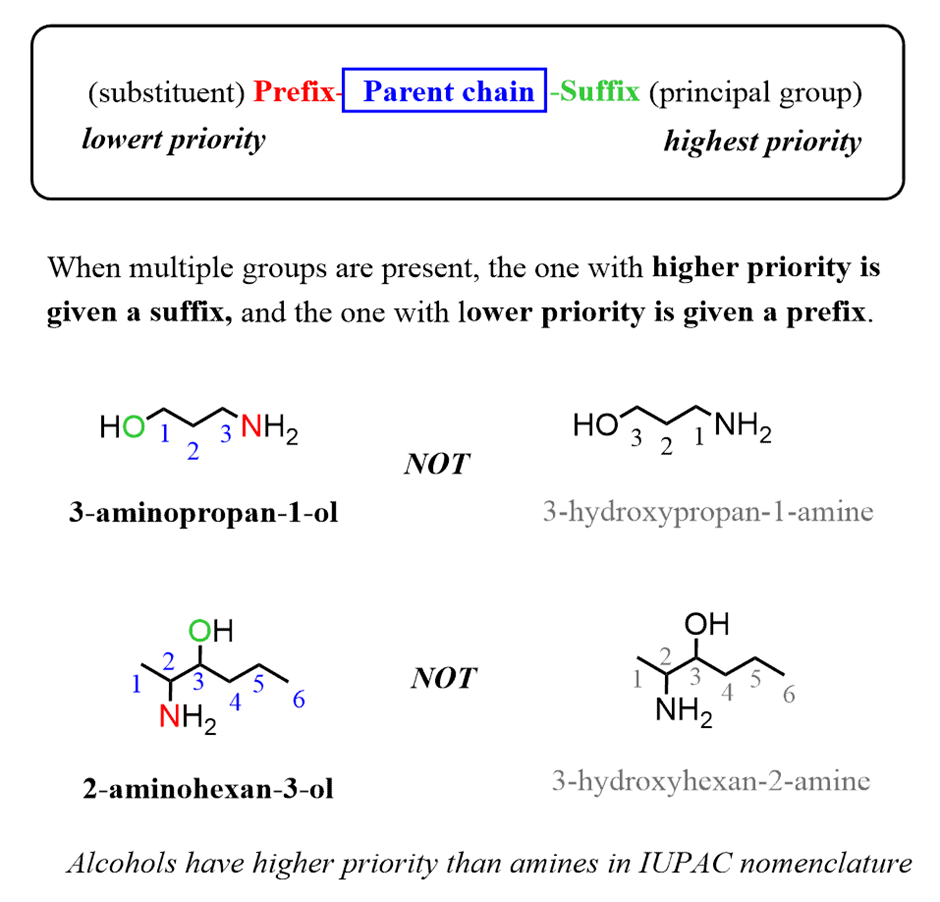
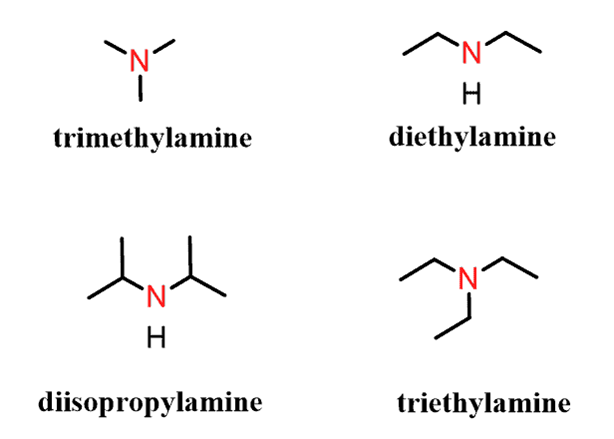
There are two case-scenarios here – all the alkyl groups connected to the nitrogen are the same and not all the alkyl groups are the same.
When the alkyl groups are identical, they are listed with a prefix “di” or “tri” and the compound is named just like what we have seen in the common names. We name them like alkyl amines:
If the secondary or a tertiary amine has more than one type of alkyl group, then it is named as a primary amine. The parent chain is the longest chain bonded to the amine, and the other groups are named as substituents connected to the nitrogen and preceded by an “N” (in italics). This emphasizes that they are bonded to the nitrogen rather than to a carbon:
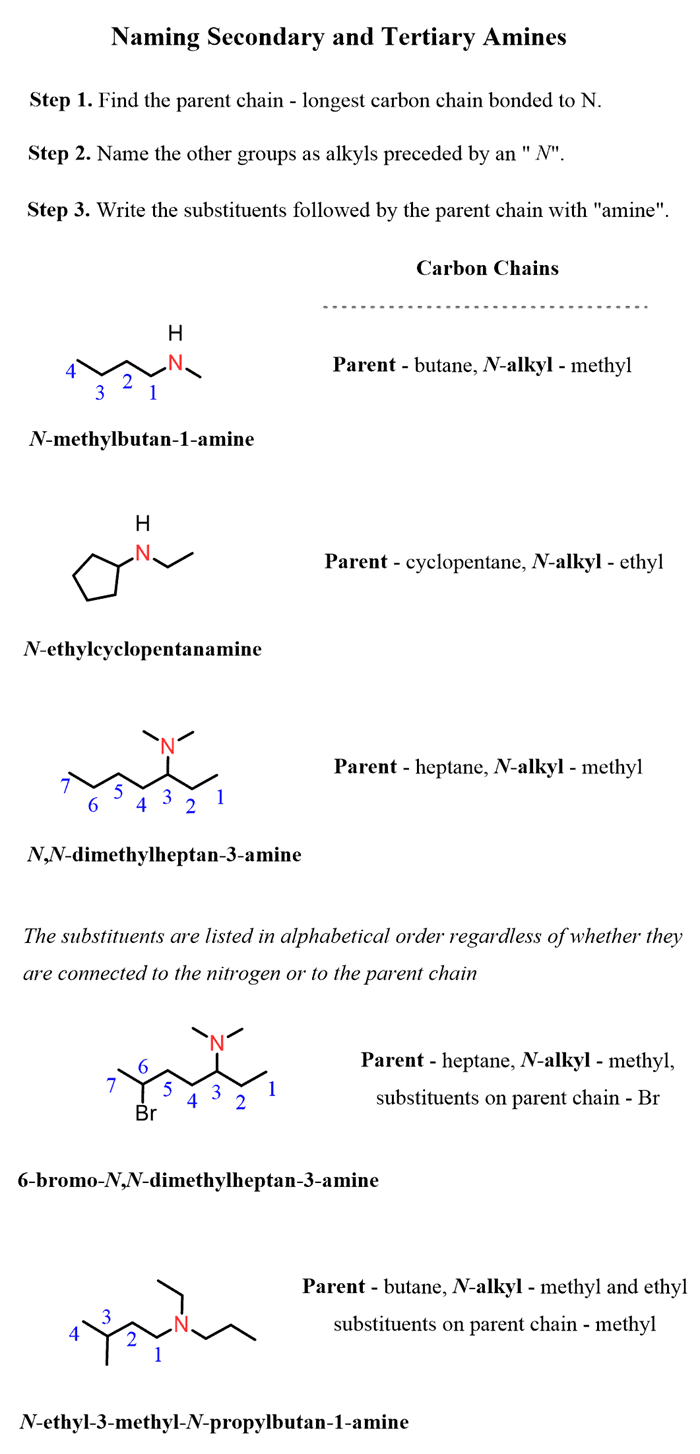
And, in the last example, the order of alkyl groups goes fromnitrogen (ethyl),parent chain (methyl), andnitrogen (propyl)which shows once again that we are prioritizing them in the alphabetical order.
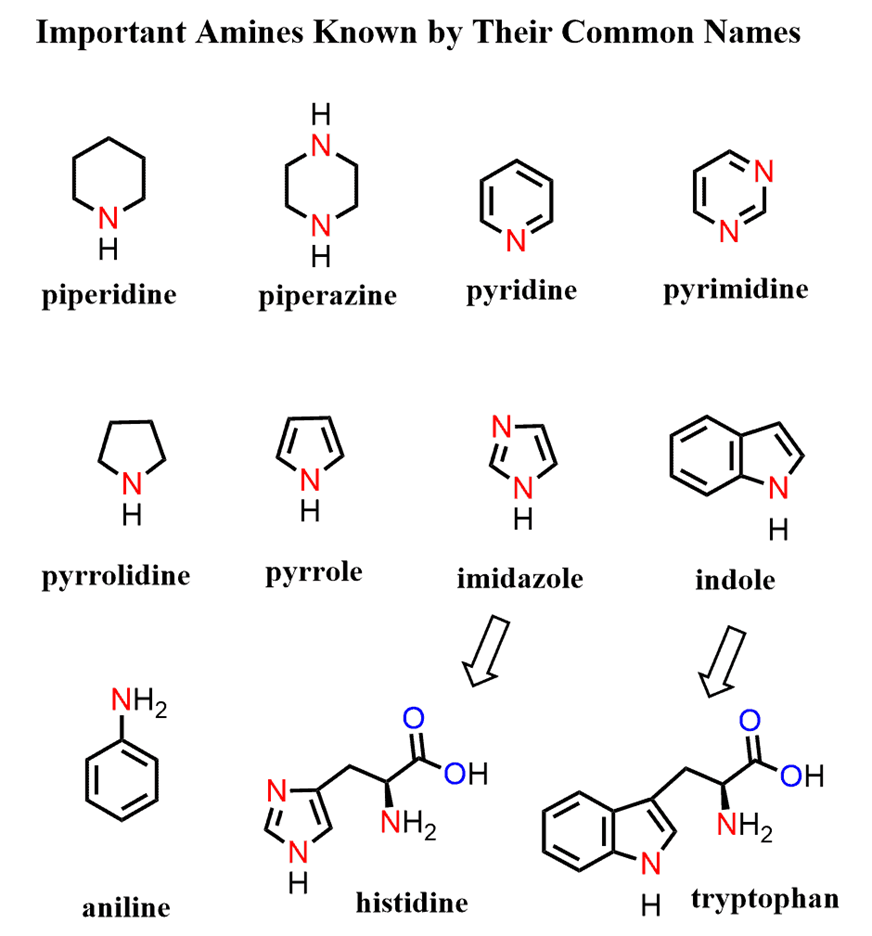
There are lots of amines that are simply referred to by their common names. Most of these are used as organic bases and constitute a part of biologically active and essential compounds such as amino acids, nitrogen-containing pain killers (alkaloids), as well as synthetic medicines.
In the IUPACnomenclature of carboxylic acids, we learned that their salts are named by replacing the suffix “ic acid” or “oic acid” with “ate”. For example, sodium acetate, potassium butyrate, etc.
The good is that esters follow the same pattern and instead of the metal ion, we use the alkyl group connected to the RCO (acyl) fragment.
For example:
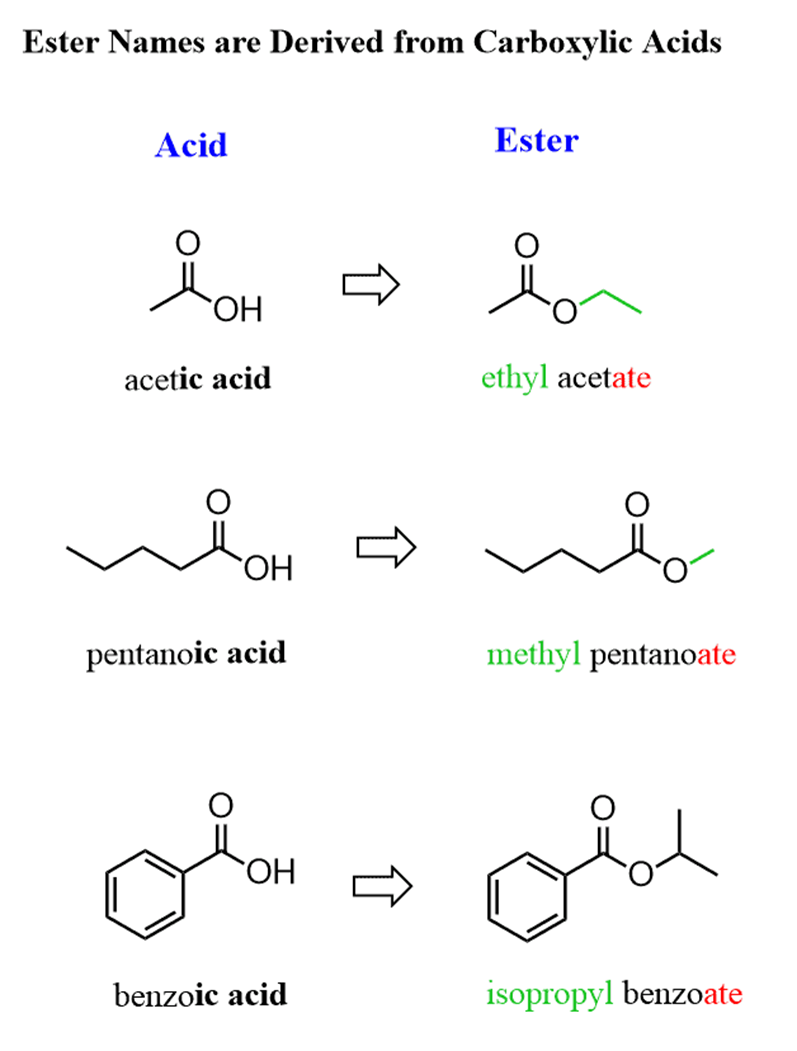
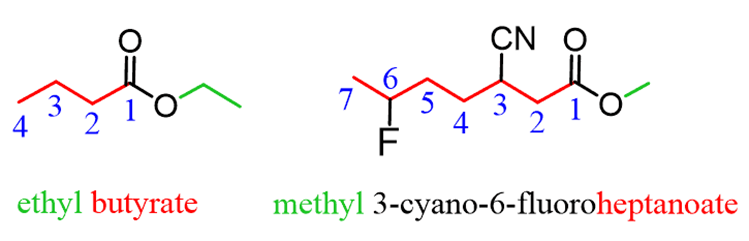
The substituents are numbered based on the position of the COOR group and placedin alphabetical order:
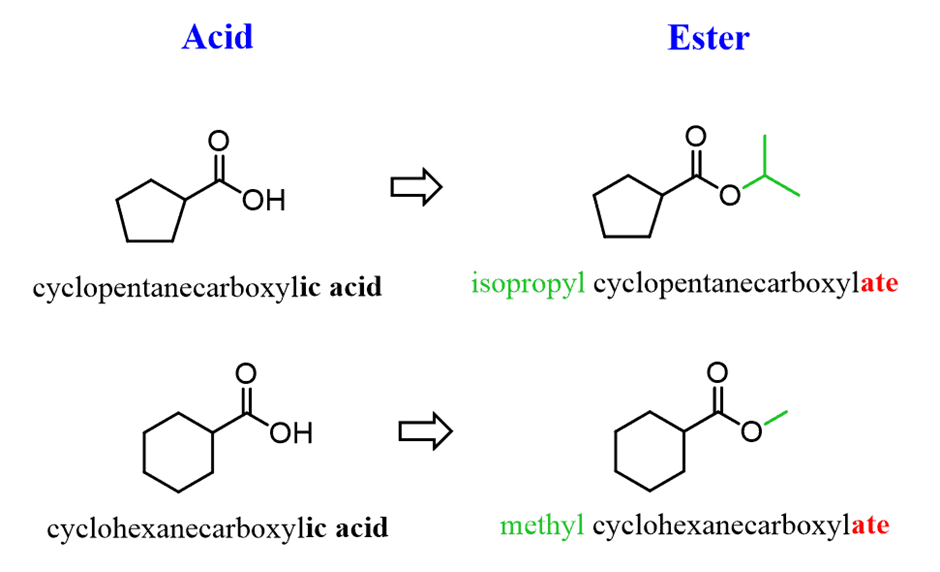
Naming Esters on a Ring
To name an ester on a ring, we need to refer to the corresponding carboxylic acid. For example, the suffix of cyclopentanecarboxylic acid is changed to carboxy”late” and the alkyl group is added at the beginning:
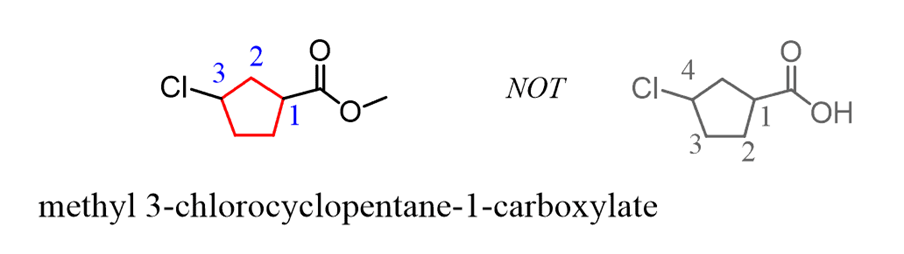
If substituents are also present, the numbering starts from the carbon connected to the COOH groupand goes in the direction thatminimizes the numberingof the substituents:
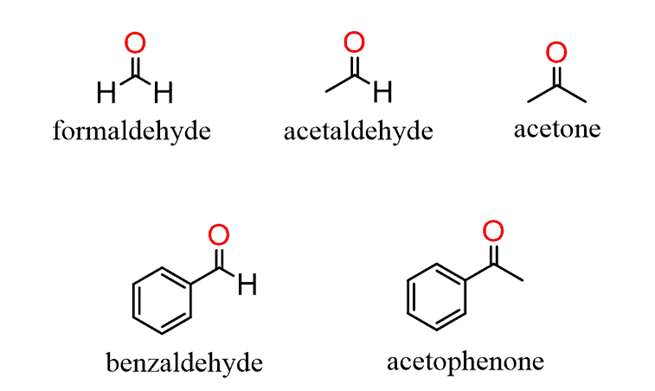
For naming aldehydes, the IUPAC nomenclature is more precise than using common naming. In IUPAC naming, the “-e” suffix is removed from the parent alkane chains and replaced with “-al”. However, if the aldehyde attaches to a ring, the “-carbaldehyde” suffix adds to the parent alkane name instead. The functional group of an aldehyde is always located at the lowest possible position number, so it is not included in the name.
Some examples of aldehydes:
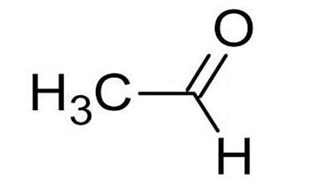 Ethanal
Ethanal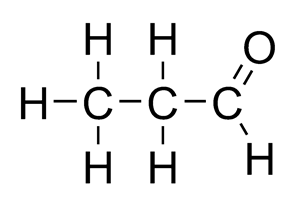 Propanal
Propanal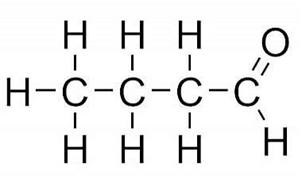 Butanal
Butanal
Nomenclature of ketone
Ketones have lower priority than aldehydes. However, if it happens to be that theketone is the highest priority in the molecule, then thesuffix changes to “one”.
So, to name a ketone, we need to choose theparent chainsuch that it is thelongest carbon chain contains the C=O group:
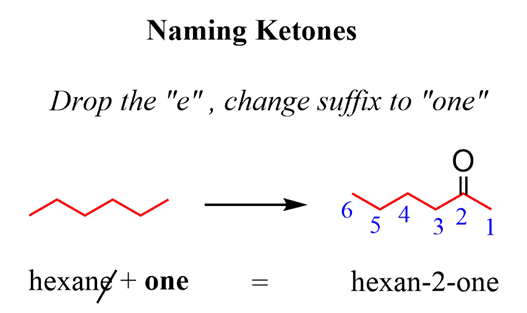
When naming acyclic ketone, start numbering the ringbeginning with the carbon connected to the C=O group. This rule always puts the =O group at C1,therefore, the “1” is usually omitted from the name:
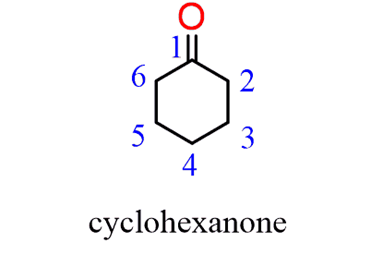
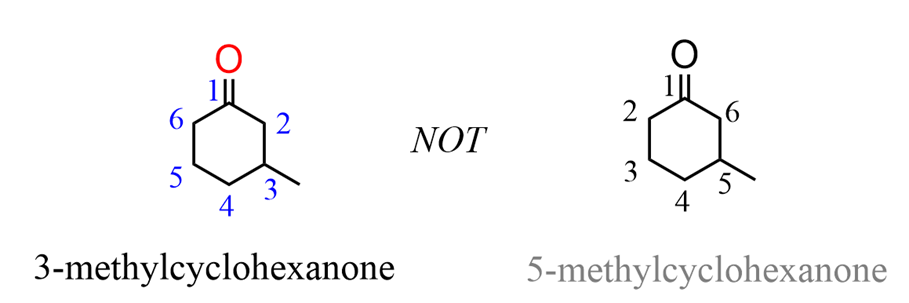
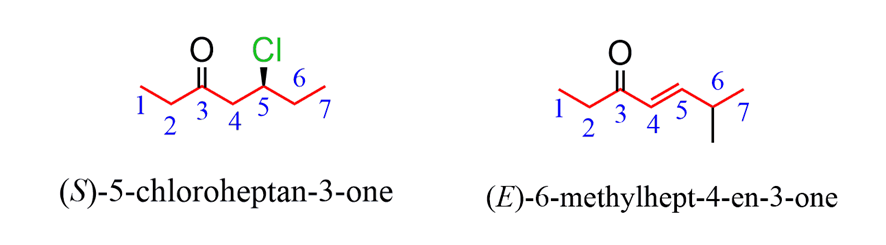
In the IUPAC nomenclature, carboxylic acids are named by adding a suffix to the parent name of the longest chain. If the parent chain is noncyclic, you need to first find thelongest carbon chain containing the -COOHgroup andchange the suffixfrom “ane” to “oic acid” dropping the “e” and the locant “1” in the final name:
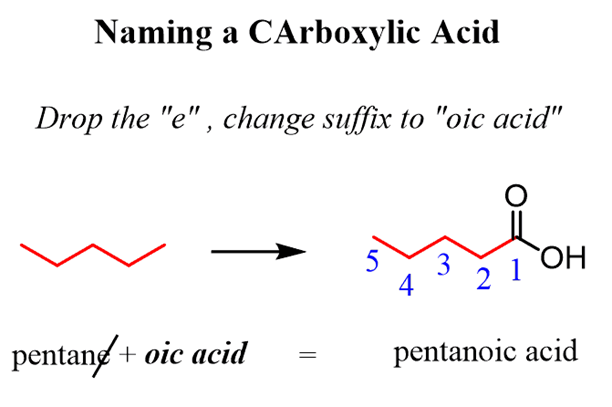
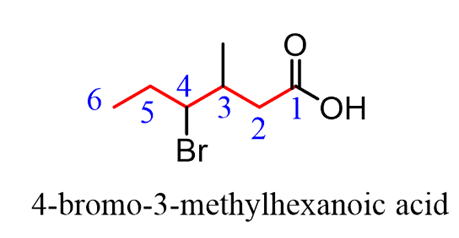
The substituents are numbered based on the position of the COOH group and placedin alphabetical order:
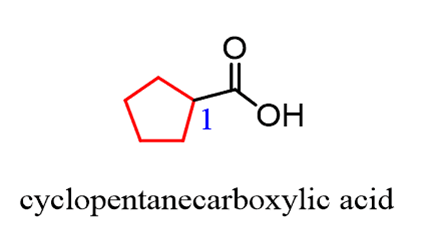
The only out-of-normal situation you may encounter is when the-COOH group is on a ring. In this case, we name the ring and add the words “carboxylic acid”:
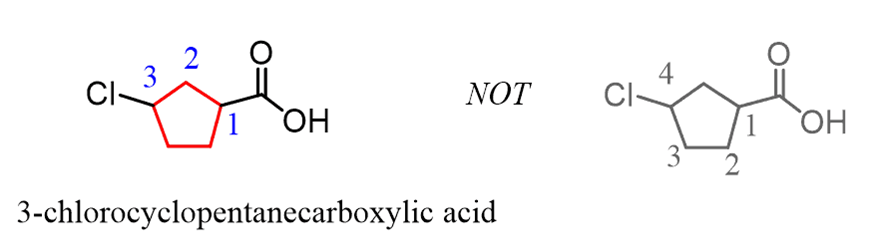
Naming Carboxylic Acids with Functional Groups
Carboxylic acids have higher prioritythan all the otherfunctional groups andtherefore, theydefine the parent chainand give the correspondingsuffixto the compound’s name. All theother groupsstanding below in the functional group priority table are added as aprefix.
As an example, let’s name this compound containing a carboxylic acid, a halide, and nitrile groups:
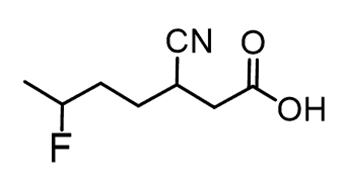
The parent chain is heptane and we have a heptanoic acid. Halogens are one of thegroupsthat are not considered in the priority list of functional groups, so they arealways substituentsand get aprefix. So, in the final name,we will simply place “fluoro” in the alphabetical order.
The nitrile group has a lower priority and will get theprefix “cyano”since it is also treated as a substituent:
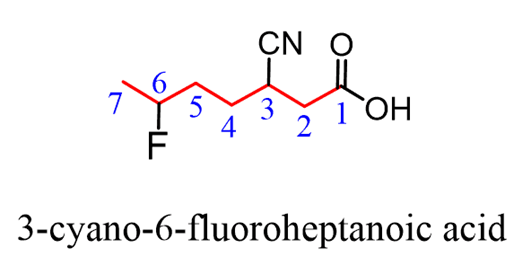
Carboxylic acids can also be identified by theircommon names.These are very common, and it would be beneficial to memorize them:
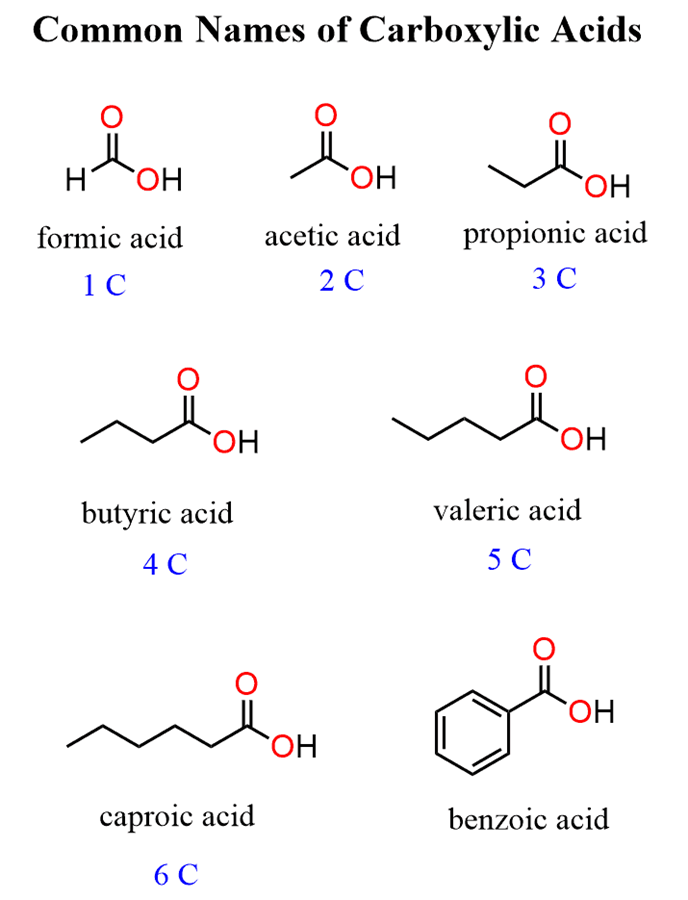
When substituted carboxylic acids are named by common names, the carbon positions are often designated with Greek letters. The carbon next to the COOH is called theɑcarbon, followed byβ,γ(gamma),δ(delta), etc. The last carbon can be referred to asW(omega) positions.
For example:
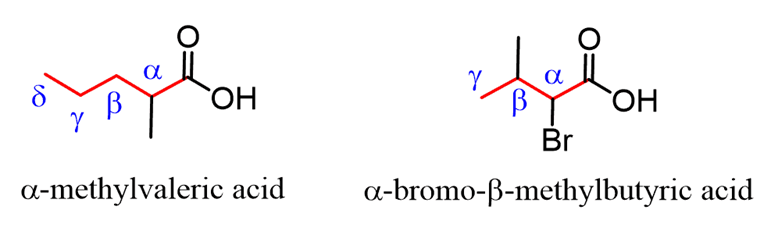
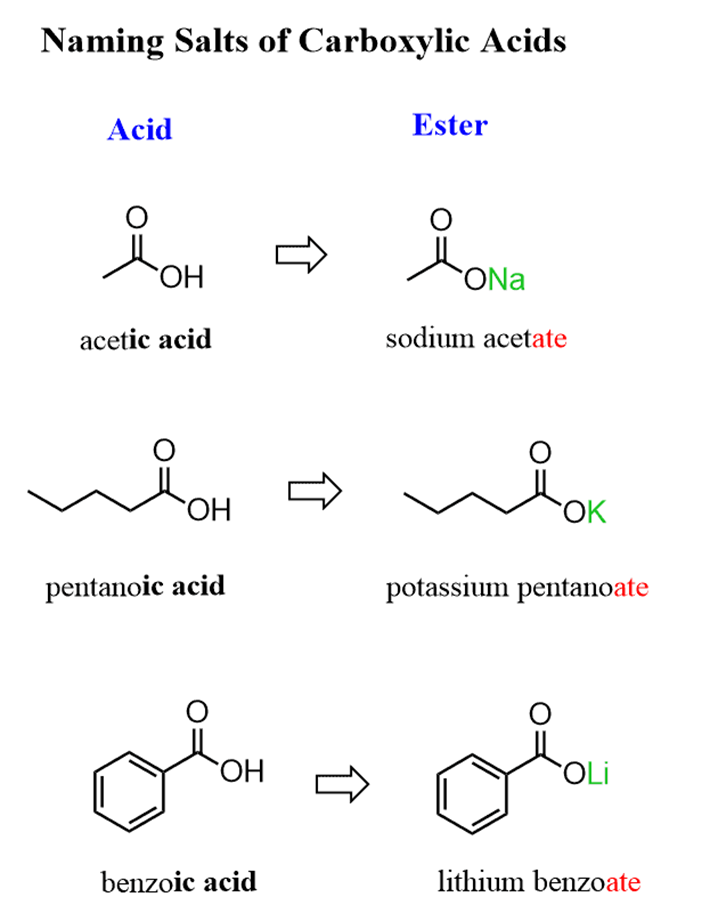
Naming Salts of Carboxylic Acids
Carboxylic acid salts are named by replacing the suffix “ic acid” or “oic acid” with “ate”. For example:
 Nomenclature of acid anhydride and nitriles
Nomenclature of acid anhydride and nitriles
A series of linear acid anhydrides is shown below with the molecular structure of each acid anhydride in the series drawn out in full (showing bond types but not accurate bond angles or, therefore, realistic molecular shapes).
|
Carbon atoms in chain |
Name and simple formula |
Simple Structure showing bond types but not accurate bond angles |
Examples of other names (synonyns) |
|
|
|||
|
1 |
formic anhydride |
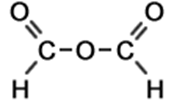 |
|
|
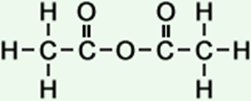
2 |
ethanoic anhydride |
 |
|
|
3 |
propanoic anhydride |
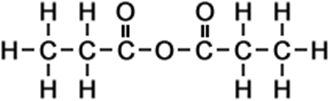 |
|
|
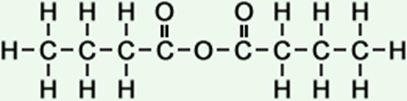
4 |
butanoic anhydride |
 |
|
|
5 |
pentanoic anhydride |
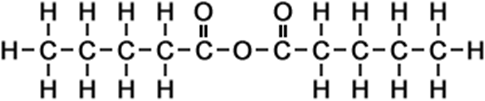 |
|
|
6 |
hexanoic anhydride |
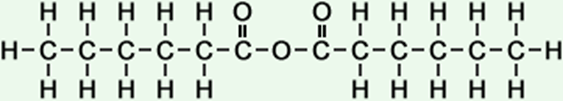 ·hexanoic acid anhydride ·caproic anhydride |
|
|
7 |
heptanoic anhydride |
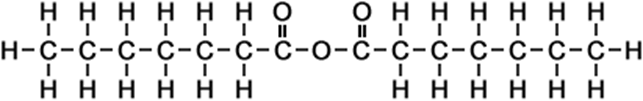 ·n-heptanoic anhydride ·heptanoic acid anhydride ·n-heptanoic acid anhydride ·enanthic anhydride ·heptanoyl anhydride |
|
|
8 |
octanoic anhydride |
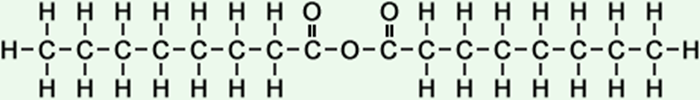 ·octanoic acid anhydride ·n-octanoic anhydride ·caprylic anhydride ·n-caprylic anhydride ·octanoyl octanoate |
|
|
9 |
nonanoic anhydride |
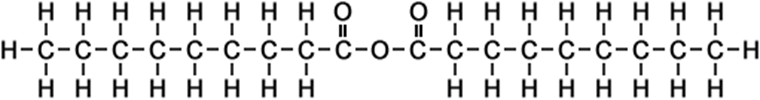 ·nonan-1-oic anhydride ·pelargonic anhydride |
|
|
10 |
decanoic anhydride |
 ·decanoic acid anhydride ·n-decanoic anhydride ·capric anhydride ·n-capric anhydride ·decanoyl decanoate |
|
Nomenclature of acid chlorides
The first ten members of thehomologous seriesof linear acid chlorides are represented below. The simple structures drawn below show bondtypessuch as single and double bonds, but not accurate bondangles.
|
Carbon atoms in chain |
Name and simple formula |
Simple Structure showing bond types but not accurate bond angles |
Examples of other names (synonyns) |
|
|
|||
|
1 |
formyl chloride (CHClO) CHOCl |
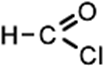 |
|
|
2 |
ethanoyl chloride (C2H3ClO) CH3COCl |
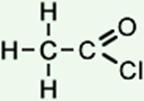 |
|
|
3 |
propanoyl chloride (C3H5ClO) CH3CH2COCl |
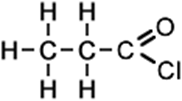 |
|
|
4 |
butanoyl chloride (C4H7ClO) CH3CH2CH2COCl |
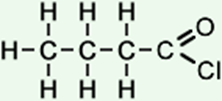 |
|
|
5 |
pentanoyl chloride (C5H9ClO) CH3CH2CH2CH2COCl |
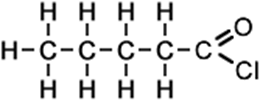 |
|
|
6 |
hexanoyl chloride (C6H11ClO) CH3CH2CH2CH2CH2COCl |
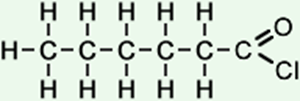 |
|
|
7 |
heptanoyl chloride (C7H13ClO) CH3CH2CH2CH2CH2CH2COCl |
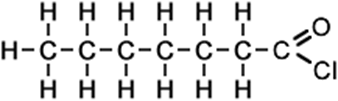 |
|
|
8 |
octanoyl chloride (C8H15ClO) CH3CH2CH2CH2CH2CH2CH2COCl |
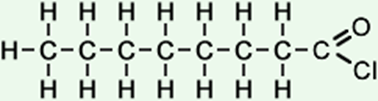 |
|
|
9 |
nonanoyl chloride (C9H17ClO) CH3CH2CH2CH2CH2CH2CH2CH2COCl |
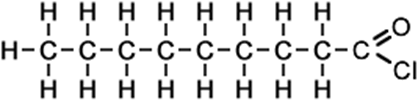 |
|
|
10 |
decanoyl chloride (C10H19ClO) CH3CH2CH2CH2CH2CH2CH2CH2CH2COCl
|
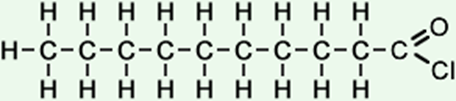 |
|
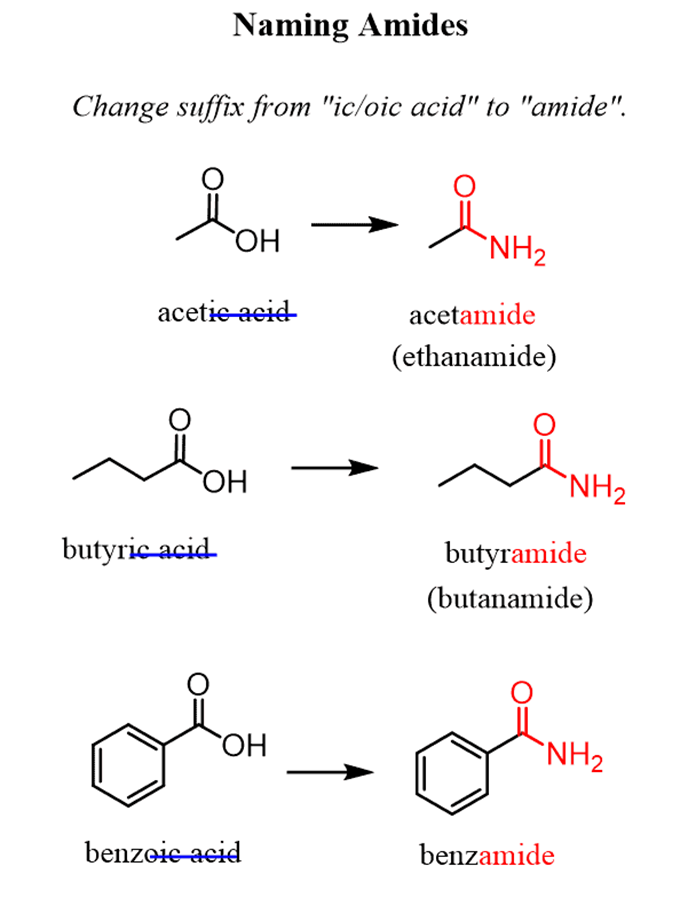
Nomenclature of amides
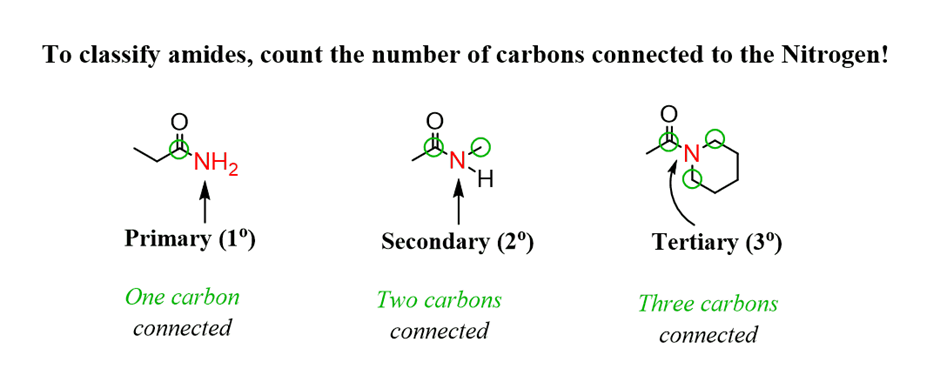
For example:
All the substituents are numbered by starting from the amide carbon (unless ahigher priority groupis present) and placed alphabetically just like for naming any other functional group:
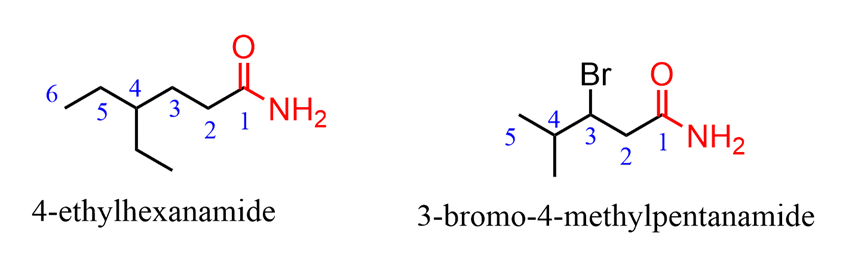
When the amide group is connected to a ring, the suffix “carboxylic acid” is replaced with “carboxamide”:
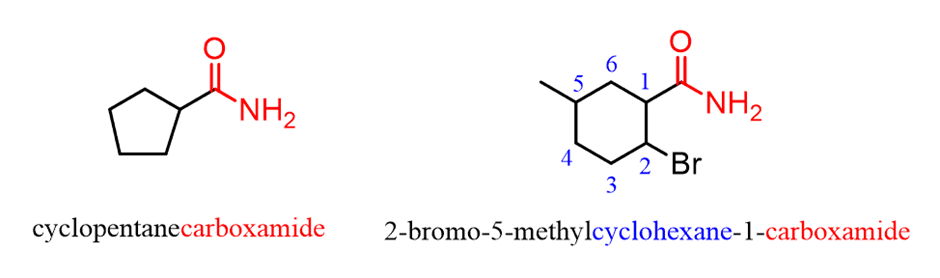
Notice that the amide carbon, in this case, is not counted as part of the parent chain.
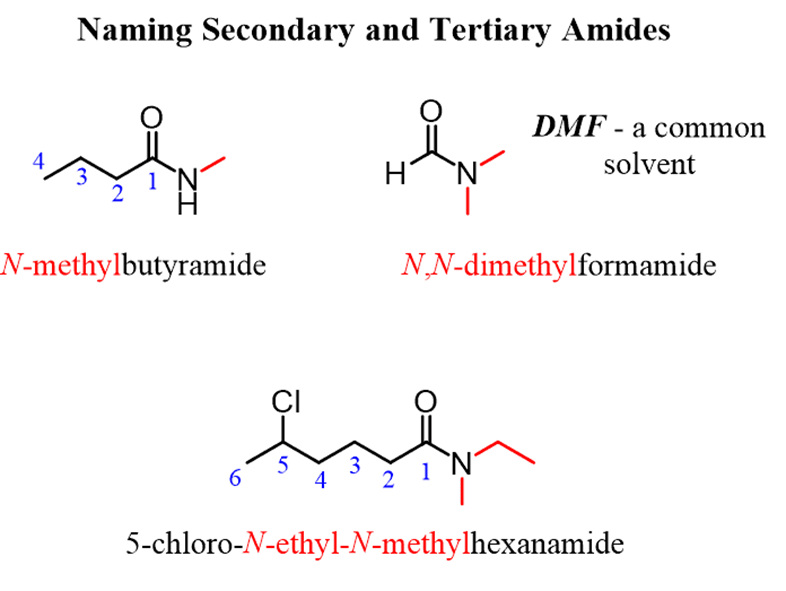
Secondary and tertiary amides bearalkyl group(s) on the nitrogenand just like other substituents, these are placed at the beginning of the name. However, these alkyl groups are also specifically indicated with theletter “N”.
For example,N-methyl,N-ethyl, orN-ethyl, N-propyl if two alkyl groups are connected:
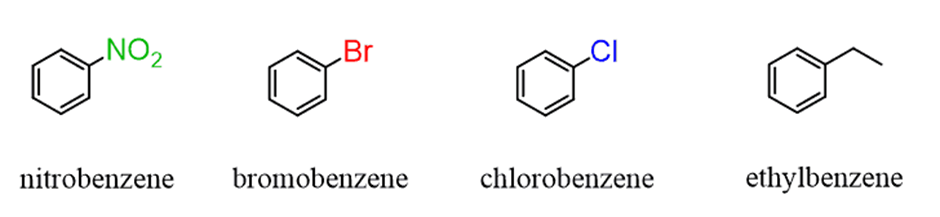
Nomenclature of Substituted benzene compounds
Nomenclature of aromatic hydrocarbon
Benzene is the “first and simplest” aromatic compound and many monosubstituted derivatives of benzene are named systematically by adding thename of the substituent to “benzene” which is the parent:For examples:
If the substituent is an alkyl chain with more carbon atoms than benzene, then benzene can be treated as a substituent. The ring, in this case, is called a phenylgroup just like the methyl, ethyl, etc. The phenyl group is often abbreviated as “Ph”.
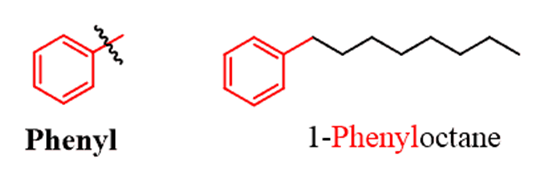
There are also monosubstituted benzene rings that have common names. And when naming an aromatic compound with one of these rings,you need to use the common name as the parent and not the “benzene”. Below is the list of these common names:
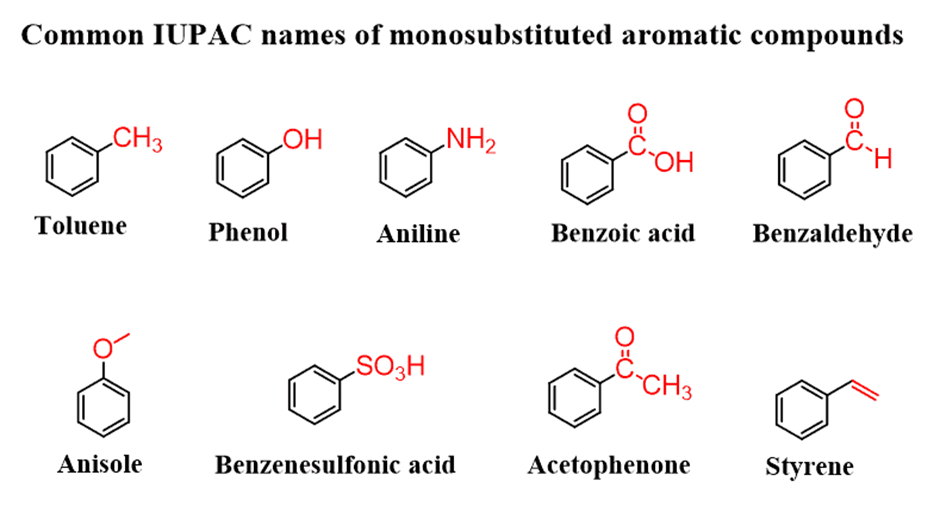
Unfortunately, there are no other options and you need to memorize these names. At least the first row since they are more common, and you will encounter them all when dealing with aromatic compounds.
Some disubstituted benzene rings also have common names, and the first thing here is to know the relative positions ofortho,meta,andpara:
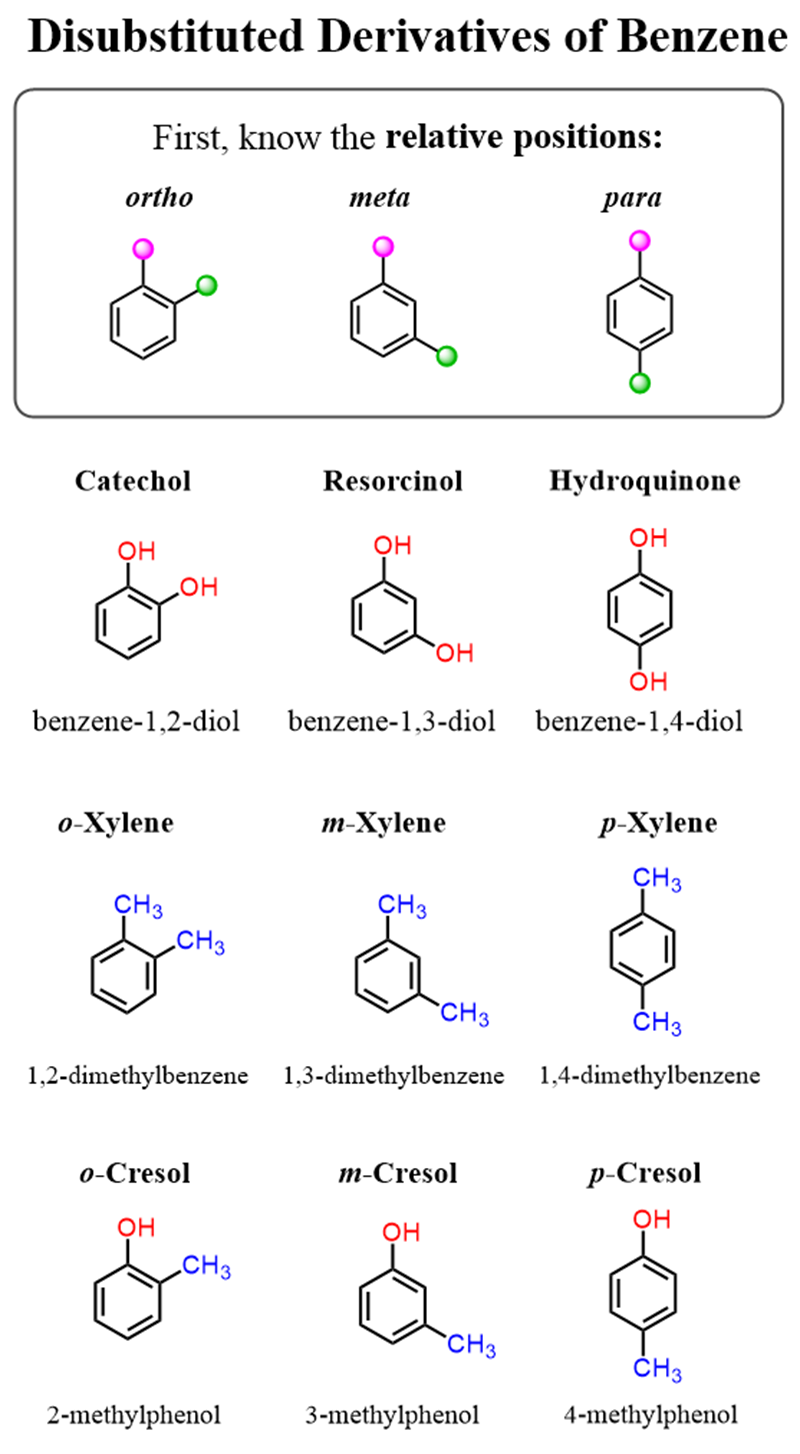
For the other rings with common names, start numbering the ring from the substituent that is part of the common ring such that the other groups get the smallest possible locants:
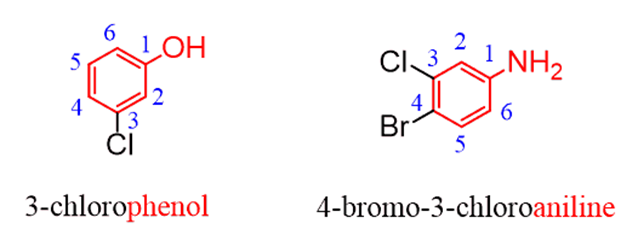
Using “benzene” as the parent can also be encountered, even though it is not what you’d commonly see:
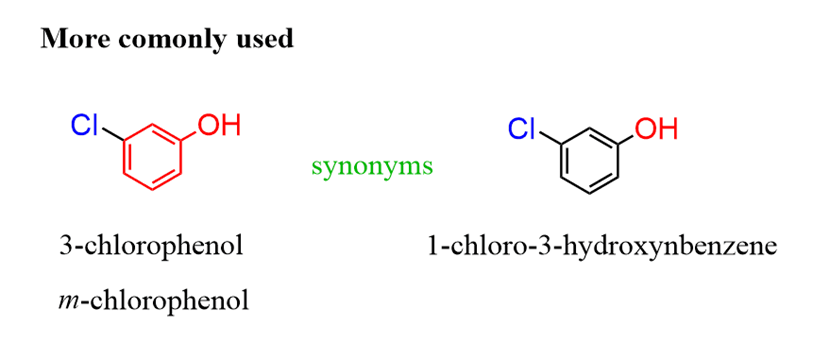
In general, to name an aromatic compound, you can follow these steps:
1. Identify and name the parent. If it is not one of the common names, then use benzene.
2. Identify and name the substituents.
3. Number the ring to give the substituents the smallest possible number.
4. Put the substituents alphabetically followed by the parent name.
For example, in ortho-dibromobenzene, numbering from the top Br goes clockwise to have a 1,2, instead of 1,6 locant order:
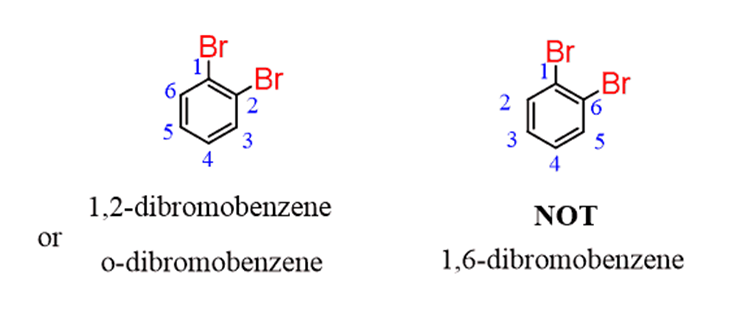
We do the same way if there are more substituents:

If the numbering does not make a difference, then give a smaller locant to the alphabetical priority:
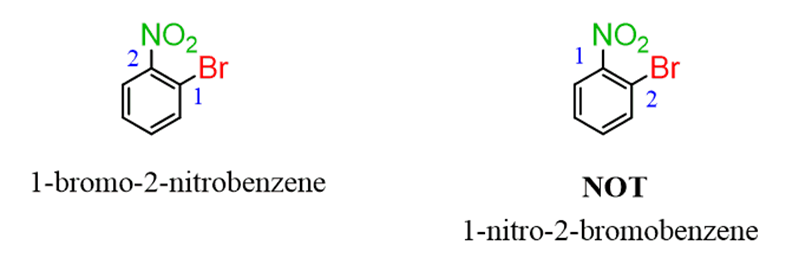
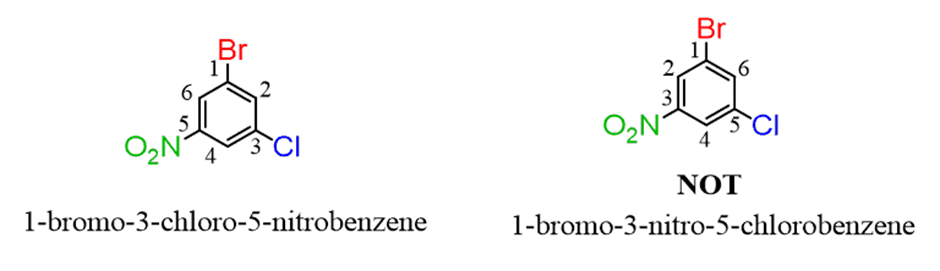
If there are more than two groups, and numbering doesn’t make a difference, start from the alphabetical priority and number the ring toward the next alphabetical priority:
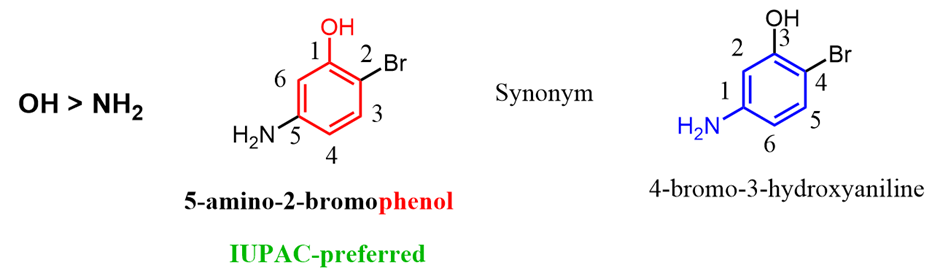
In reference to the question asked in the comments, I also wanted to address the situation whentwo common names are possible. For example phenol and aniline, we need to go based on thepriority of the groups. In this case, we are comparing the amine and alcohol, and sinceOH stands above NH2in the priority chart, the parent chain should be an alcohol (phenol) rather than aniline.