BASIC CONCEPT OF ORGANIC CHEMISTRY
BONDING AND SHAPES OF ORGANIC COMPOUNDS
Shapes of Carbon Compounds
The knowledgeof fundamental concepts of molecular structure helps in understanding and predicting the properties of organic compounds. It may be recalled that formation and the shapes of molecules like methane (CH4), ethene (C2H4), ethyne (C2H2) are explained in terms of the use ofsp3,sp2andsphybrid orbitals by carbon atoms in the respective molecules.
Hybridisation influences the bond length and bond enthalpy (strength) in compounds. Thesphybrid orbital contains morescharacter and hence it is closer to its nucleus and forms shorter and stronger bonds than thesp3hybrid orbital. Thesp2hybrid orbital is intermediate inscharacter betweenspandsp3and, hence, the length and enthalpy of the bonds it forms, are also intermediate between them. The change in hybridisation affects the electronegativity of carbon. The greater thescharacter of the hybrid orbitals, the greater is the electronegativity. Thus, a carbon atom having ansphybrid orbital with 50%scharacter is more electronegative than that possessingsp2orsp3hybridised orbitals.
Some Characteristic Features ofπBonds
In aπ(pi) bond formation, parallel orientation of the twoporbitals on adjacent atoms is necessary for a proper sideways overlap. Thus, in H2C=CH2molecule all the atoms must be in the same plane. Theporbitals are mutually parallel and both theporbitals are perpendicular to the plane of the molecule. Rotation of one CH2fragment with respect to other interferes with maximum overlap ofporbitals and, therefore, such rotation about carbon-carbon double bond (C=C) is restricted. The electron charge cloud of theπbond is located above and below the plane of bonding atoms. This results in the electrons being easily available to the attacking reagents. In general,πbonds provide the most reactive centres in the molecules containingmultiple bonds.
STRUCTURAL REPRESENTATIONS OF ORGANIC COMPOUNDS
Complete, Condensed and Bond-line structural formulas
Complete and Condensed Formulae
Each pair of electron making a covalent bond is represented by a dash (—).
Two dashes represent a double bond and three dashes represent a triple bond.


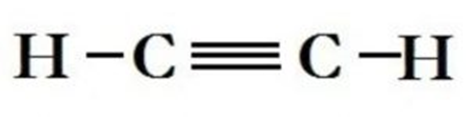

Such structural representation are calledcomplete structural formulae or graphic or displayed formulae.
These structural formulae can be further condensed by enclosing the repetitive structural unit within a bracket and placing an integer as a subscript indicating the number times the structural unit gets repeated.
For Ex :CH3CH2CH2CH2CH2CH2CH3can be condensed to CH3(CH2)5CH3
CH3CH2CH2CH2CH2CH2CH2COOHcan be condensed to CH3(CH2)6COOH
1) It is a simple , short and convenient method of representing organic molecule.
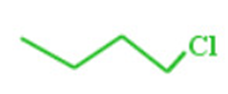
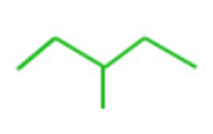
2) Carbon-carbon bonds are shown by lines drawn in a zig-zag fashion and carbon atoms by line ends and intersection.
3)A single bond is represented by a single line , a double bond by 2 parallel lines and a triple bond by three parallel lines.
4) Carbon atoms are not shown but all atoms other than carbon and hydrogen atoms such as oxygen, nitrogen, halogen are shown on zig-zag line.
5) Each carbon on the line end or intersection is attached to required number of hydrogen atoms i.e. terminal denote CH3group and an unsubstituted intersection denotes a CH2group.
For Ex: butyl chloride
3-methyl pentane
Polygon formulae
There are many organic compounds ,in which carbon atoms are not joined in a chain but are joined in a ring.These are calledcyclic compoundsand are usually represented by polygon without showing carbon and hydrogen atoms.
The corners of a polygon represent a carbon atom and the sides of a polygon denote a carbon-carbon bond.
If an atom or a group of atoms other than hydrogen is attached to carbon, then that atom or a group of atoms is shown in this structure.
For Ex: Cyclopropane

Cyclobutane

Cyclopentane

Cyclohexane

chlorocyclohexane

Three-dimensional representation of organic molecules
1) The three-dimensional structure of organic compound can be represented on paper by using certain conventions.
2) By using solid and dashed wedge formula, 3-D image of a molecule from a two-dimensional picture can be perceived.
3) The thick solid line or the solid wedge indicates a bond lying above the plane of the paper and projecting towards the observer.
4) A dashed wedge is used to represent a bond lying below the plane of paper and projecting away from the observer.
5) Wedges are used in such a way that the broad end is towards the observer.The bond lying in the plane of paper are depicted by using a normal or an ordinary line.
A representation which completely describes the actual positions of various atoms of a molecule in space is called aspatial formulae or three-dimensional i.e. 3-D structure.
