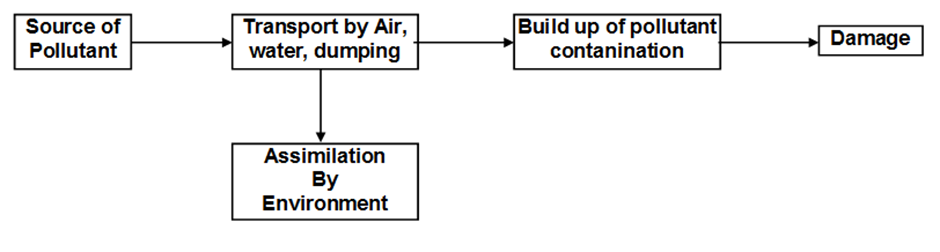
Atmospheric pollution
- Atmospheric pollution is generally as tropospheric and stratospheric pollution.
Process of contamination of the environment with harmful wastes arising mainly from human activities.
- Pollutant:Any substance or species produced either by a natural source or by human activity, which produces adverse effect on the environment.
- Contaminant:A substance which does not occurs in nature but is introduced by human activity into the atmosphere affecting its composition.
- Source:The site from which the pollution or contaminants originate.
- Sink:The material or medium which consumes or interacts with a long lived pollutant is called sink.
- Receptor: Anything that is affected by the pollutants.
- Threshold limit value (TLV) :This indicates the permissible limit of a pollutant in atmosphere to which a healthy worker is exposed during hours a day or 40 hours a week for life time without any adverse effects. TLV are determined by experimentation on animals, by use of medical knowledge, epidemiology surveys & environmental studies.
Tropospheric pollution-Tropospheric pollutionoccurs due to the presence of undesirable solid or gaseous particles in the air.
The following are the major gaseous and particulate pollutants present in the troposphere:
- Gaseous air pollutants:These are oxides of sulphur, nitrogen and carbon, hydrogen sulphide, hydrocarbons, ozone and other oxidants.
- Particulate pollutants:These are dust, mist, fumes, smoke, smog etc.
|
Gaseous air pollutant |
|
|
Oxides of sulphur - Oxides of sulphur are produced when sulphur containing fossil fuel is burnt. |
|
|
Oxides of Nitrogen – produced from combustion of coal, gasoline, natural gas, petroleum refining, chemical plants, manufacturing explosives and fertilizers, tobacco smoke. |
The irritant red haze in the traffic and congested places is due to oxides of nitrogen. Higher concentrations of NO2damage the leaves of plants and retard the rate of photosynthesis. Nitrogen dioxide is a lung irritant that can lead to an acute respiratory disease in children. It is toxic to living tissues also. Nitrogen dioxide is also harmful to various textile fibres and metals. |
|
Hydrocarbons: they are formed by incomplete combustion of fuel used in automobiles. |
Hydrocarbons are carcinogenic, i.e., they cause cancer. They harm plants by causing ageing, breakdown of tissues and shedding of leaves, flowers and twigs. |
|
Oxides of Carbon-carbon monoxide The polluted gas emitted from the vehicles is mainly Carbon monoxide. Other sources, which produce CO, involveincomplete combustion of coal, firewood, petrol, etc. |
Carbon monoxide is ahighly poisonous gas.If inhaled, it causes nausea, headache and giddiness. Inhalation of carbon monoxideleads to death in a few minutes. Carbon monoxidecombineswithhaemoglobin and decreases its oxygen-carrying capacity. As a result, the blood becomes oxygen-deficient and causes unconsciousness or death. In pregnant women who have the habit of smoking the increased CO level in blood may induce premature birth, spontaneous abortions and deformed babies. |
|
Carbon dioxide – Carbon dioxide is released into the atmosphere by respiration, burning of fossil fuels for energy, and by decomposition of limestone during the manufacture of cement. It is also emitted during volcanic eruptions. |
The increased amount of CO2in the air is mainly responsible for global warming. Carbon dioxide gas is confined to troposphere only. |
|
Particulate pollutants – Particulate pollutants causes allergic reactions, reduces visibility by producing haze in the atmosphere. Smoke blacken the buildings and our clothes. |
|
|
Smoke- Examples are cigarette smoke, smoke from burning of fossil fuel, garbage and dry leaves, oil smoke etc. Dust- Blasting, Stone crushers, Woodwork. Cement- Cement industry, Construction of buildings Asbestos-Asbestos mining, Manufacture of asbestos sheets and fire-proof fabric etc. Lead oxide- Tetraethyl lead in petrol. Mercury- Mining, Use of fungicides. Unburnt hydrocarbons - Incomplete combustion of petrol and diesel. Aerosol - Fine particles of solids and liquids, e.g., smoke and fog. Fly-ash - Small ash particles of the fuel like coal carried into the air by gases. Fog - Minute water particles suspended in air near to the surface of earth. It occurs during the winter season. Fumes- Minute solid particles formed by the condensation of vapours during sublimation, distillation etc. Mist- Suspension of liquid particles in the air, which arise from chemical reactions. Droplets- Small particles of water or any other liquid suspended in air in turbulent condition. |
|
Particulate matter:
- Soot:produced by incomplete combustion of carbonaceous fossils fuels such as coal, fuel oil, natural gas, wood etc in insufficient supply of oxygen.
- Metal particles:These are released by various metal finishing operation. The micro particles of toxic metal & SO2gas present in the polluted atmosphere get absorbed on the particles rendering them highly toxic.
- Metal oxides:They are generated by combustion of fuels containing metallic compounds.
- Lead salts:Their source is lead tetraethyl (Pb(C2H5)4) which is added to gasoline to improve its antiknock property. In order to avoid deposition of PbO suitable amounts of C2H4Cl2& C2H4Br2are added to gasoline along with Pb(C2H5)4.
- Fly ash:It originates from the combustion of high ash fossil. It contains partially burnt particles of the fuels.
- Asbestos dust:It originates from industrial units manufacturing asbestos sheets, gaskets ropes etc. Asbestos flowing & asbestos insulations also contribute towards asbestos dust in the atmosphere.
- Solid Hydrocarbons:These are emitted from petroleum refineries & comprise of paraffins, olefins & aromatics.
- Dust Particulates:Originate from natural, domestic, industrial or agricultural sources. These are thrown into atmosphere by volcanic eruptions, blowing of dust by wind, mining operations etc.
- Acid mist :Sulphuric acid mist is produced when SO3present in the atmosphere comes in contact with moisture. Nitric acid mist is produced when oxides of nitrogen, viz, NO & NO2, undergo the series of reactions in the atmosphere.
Harmful effects of particulates
- Effect on human beings:Affect the human respiratory system & cause several respiratory illnesses. The particles with small size are more harmful in this context. The particulates in fact, become the carriers of the toxic substances from the atmosphere to the human & cause big health hazards.
- Effect on visibility:Particulates in the atmosphere cause scattering & absorption of sunlight & reduce the visibility.
- Effect on Materials :The adverse effect of particulates on materials include corrosion of metals (when the atmosphere is humid), erosion & soiling of building, sculptures & painted surfaces & soiling of clothes & draperies.
Global warming and green house effect-
- Greenhouse gas –
- The green house gases (CO2, CH4, O3, CFC’S ) in the atmosphere form a thick cover around the earth. About 75% of the solar energy reaching the earth is absorbed by the earth surface. The IR radiations coming from sun are not absorbed by atmospheric gases but Earth absorbs these IR radiations of short wavelength. As a result of this the temperature of earth stands rising. Eventually, earth starts emitting infrared radiations of longer wavelengths. The partially radiated infrared radiations from the earth are absorbed by the greenhouse gases. This results in excessive heating of Earth’s atmosphere. Thus the greenhouse gases add to the heating of atmosphere. This causes global warming.The atmosphere traps the sun’s heat near earth’s surface and keeps it warm. The reemission of the earth’s energy absorbed by CO2and other greenhouse gases present near the earths surface and its radiation back to the earth is called green house effect.
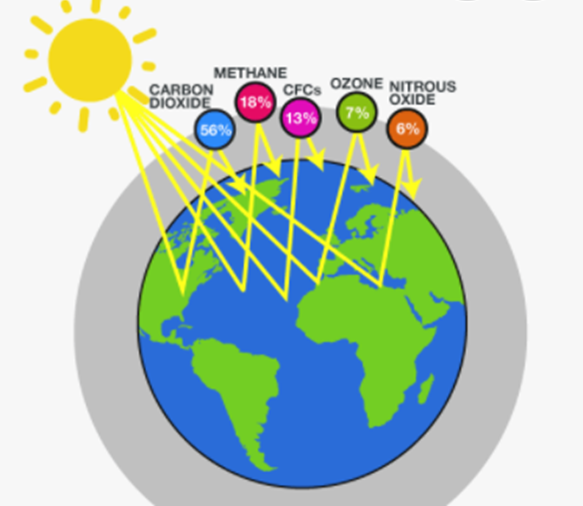
Greenhousegases:
- Carbon dioxide
- Methane
- Water vapour
- Nitrous oxide
- CFCs(Chlorofluorocarbons )
- Ozone
Advantages of green house effect:
- It is necessary for evaporation of water, formation of clouds, rainfall etc.
- The warm atmosphere helps in rapid growth of plants, trees etc.
Harmful effects of green house effect :
- High temperature of atmosphere may melt polar ice caps which are likely to raise the level of sea thereby sinking most of the coastal areas and causing large scale destruction.
- The high temperature may reduce crop product.
- The high temperature will reduce work efficiency of human being.
- Tropical rains and hurricane will become more frequent and also stronger causing more devastation.
- The change in ocean temperature will adversely affect the warm life.
Global warminḡ
- A gradual increase in the overall temperature of the earth's atmosphere, generally attributed to the greenhouse effect caused by increased levels of carbon dioxide, CFCs, and other pollutants.
Consequences of global warming:
- The average global temperature will increase to a level that lead to melting of polar ice caps as well as of other places like the Himalayan snow caps and flooding of low lying areas all over the earth.
- Increase in the global temperature increases the incidence of infectious diseases like dengue, malaria, yellow fever, sleeping sickness etc.
- This rise in temperature is leading to deleterious changes in the environment and resulting in odd climatic changes.
Control of Global warming:
- The measures include cutting down use of fossil fuel, improving efficiency of energy usage, reducing deforestation, planting trees and slowing down the growth of human population.
Acid rain –
- When the pH of the rain water drops below 5.6, it is called acid rain.
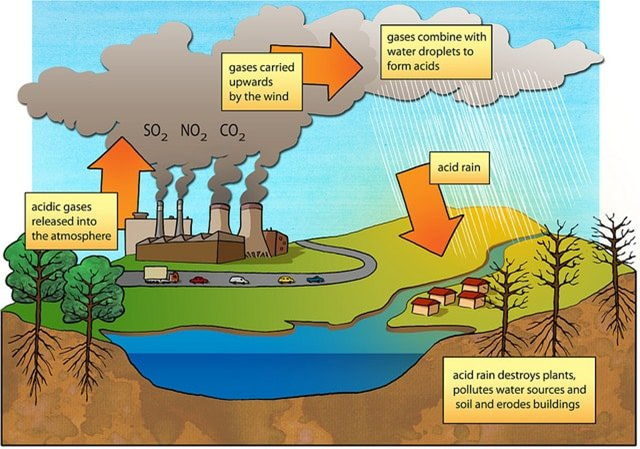
- Acid rain refers to the ways in which acid from the atmosphere is deposited on the earth’s surface.Oxides of nitrogen and sulphurwhich are acidic in nature can be blown by wind along with solid particles in the atmosphere and finally settle down either on the ground as dry deposition or in water, fog and snow as wet deposition.
- SO2and NO2after oxidation and reaction with water are major contributors to acid rain,because polluted air usually contains particulate matter that catalyse the oxidation.
Chemical reaction involved -
- 2SO2(g) + O2(g) + 2H2O (l) → 2H2SO4(aq)
- 4NO2(g) + O2(g)+ 2H2O (l) → 4HNO3(aq)
Harmful effects of acid rain -
- Acid rain is harmful for agriculture, trees and plants as it dissolves and washes away nutrients needed for their growth.
- It causes respiratory ailments in human beings and animals.
- When acid rain falls and flows as ground water to reach rivers, lakes etc. it affects plants and animal life in aquatic ecosystem.
- It corrodes water pipes resulting in the leaching of heavy metals such as iron, lead and copper into the drinking water.
- Acid rain damages buildings and other structures made of stone or metal.
- The Taj Mahal in India has been affected by acid rain.
Smog
- The smog is the combination of smoke and fog.
- Smog is air pollution that reduces visibility.
Types of smog:
- Classical smog
- Photochemical smog
|
Classical smog |
Photochemical smog |
|
This type of smog is formed due to high concentration of sulphur dioxide and particulate matter produced from fuel combination. |
This type of smog is formed due to the action of sunlight on nitrogen oxides and hydrocarbons produced by factories or automobiles. |
|
It is reducing in nature. |
It is oxidizing in nature. |
|
It occurs in cool, humid climate. |
It occurs in warm, dry and sunny climate. |
|
It can cause irritation, bronchitis and lung problem. |
It causes irritation to the eyes. |
Photochemical smog
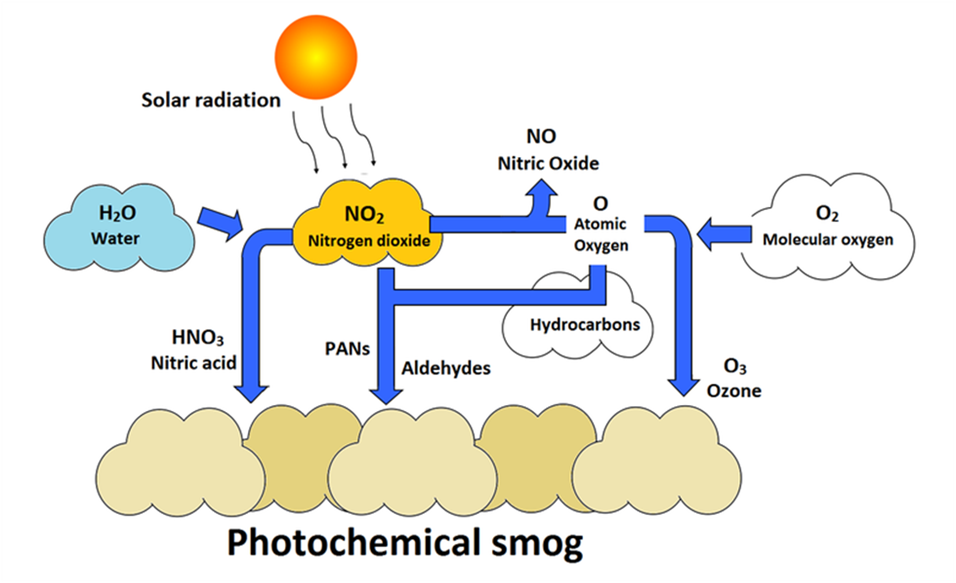
Effects of photochemical smog
- The common components of photochemical smog are ozone, nitric oxide, acrolein, formaldehyde and peroxyacetyl nitrate (PAN).
- Both ozone and PAN act as powerful eye irritants.
- Ozone and nitric oxide irritate the nose and throat and their high concentration causes headache, chest pain, dryness of the throat, cough and difficulty in breathing.
- Photochemical smog leads to cracking of rubber and extensive damage to plant life.
- It also causes corrosion of metals, stones, building materials, rubber and painted surfaces
Photochemical smog can be controlled -
- Plants e.g., Pinus, Juniparus, Quercus, Pyrus and Vitis can metabolise nitrogen oxide and therefore, their plantation help to control photochemical smog.
- If we control the primary precursors of photochemical smog, such as NO2 and hydrocarbons, the secondary precursors such as ozone and PAN, the photochemical smog will automatically be reduced.
- Usually catalytic converters are used in the automobiles, which prevent the release of nitrogen oxide and hydrocarbons to the atmosphere
Stratospheric pollution
Ozone
- The upper stratosphere consists of considerable amount of ozone (O3), which protects us from the harmful ultraviolet (UV) radiations (λ 255 nm) coming from the sun. These radiations cause skin cancer (melanoma) in humans. Therefore, it is important to maintain the ozone shield.
- Ozone is thermodynamically unstable and decomposes to molecular oxygen. Thus, a dynamic equilibrium exists between the production and decomposition of ozone molecules.
- The main reason of ozone layer depletion is believed to be the release of chlorofluorocarbon compounds (CFCs), also known as freons. These compounds are nonreactive, non flammable, non toxic organic molecules and therefore used in refrigerators, air conditioners, in the production of plastic foam and by the electronic industry for cleaning computer parts etc.